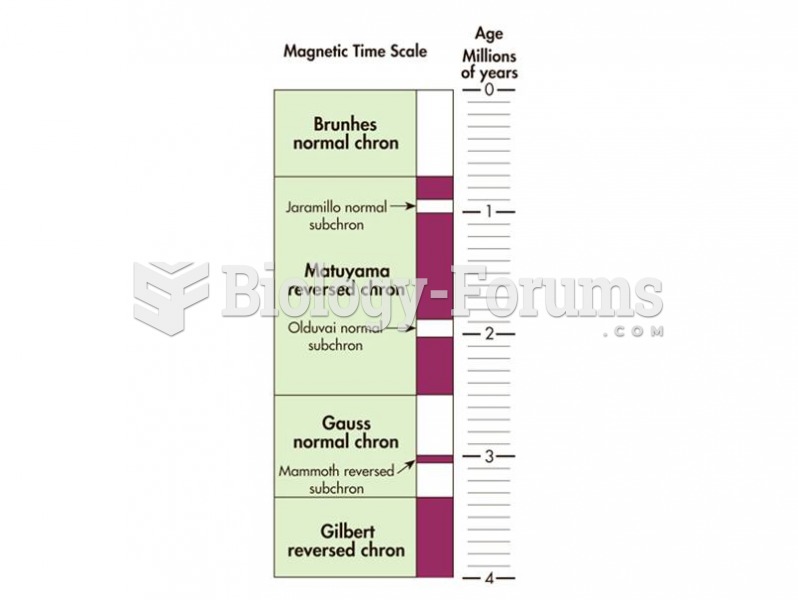
|
|
|
Vital signs (blood pressure, temperature, pulse rate, respiration rate) should be taken before any drug administration. Patients should be informed not to use tobacco or caffeine at least 30 minutes before their appointment.
To prove that stomach ulcers were caused by bacteria and not by stress, a researcher consumed an entire laboratory beaker full of bacterial culture. After this, he did indeed develop stomach ulcers, and won the Nobel Prize for his discovery.
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
A good example of polar molecules can be understood when trying to make a cake. If water and oil are required, they will not mix together. If you put them into a measuring cup, the oil will rise to the top while the water remains on the bottom.
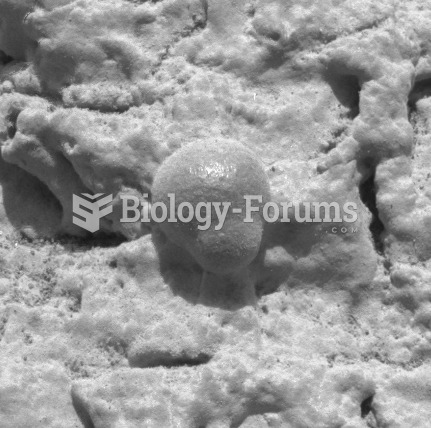 Microscopic photo taken by Opportunity showing a gray hematite concretion, indicative of the past pr
Microscopic photo taken by Opportunity showing a gray hematite concretion, indicative of the past pr
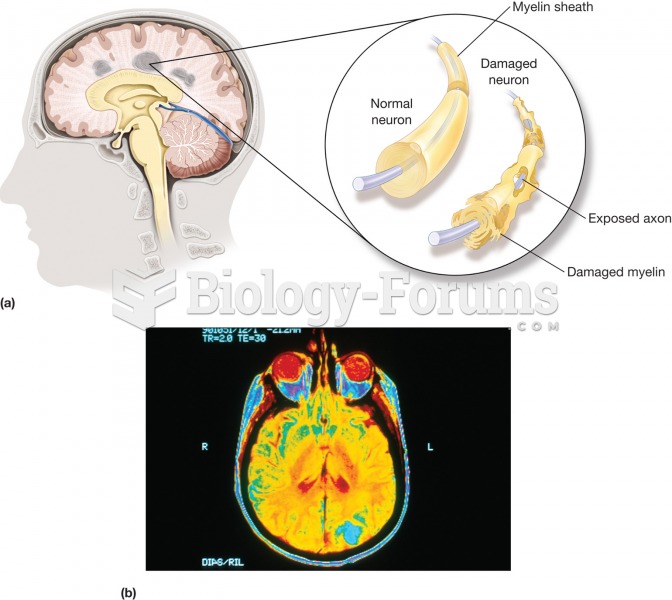 Multiple sclerosis (MS). (a) A disease characterized by the gradual development of small areas of ha
Multiple sclerosis (MS). (a) A disease characterized by the gradual development of small areas of ha