This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
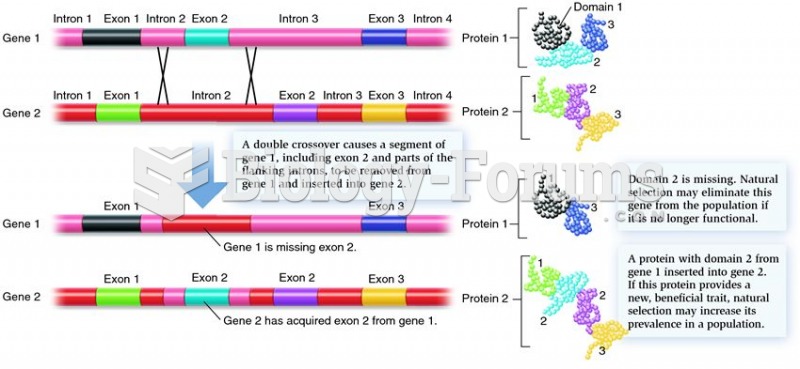
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Did you know?
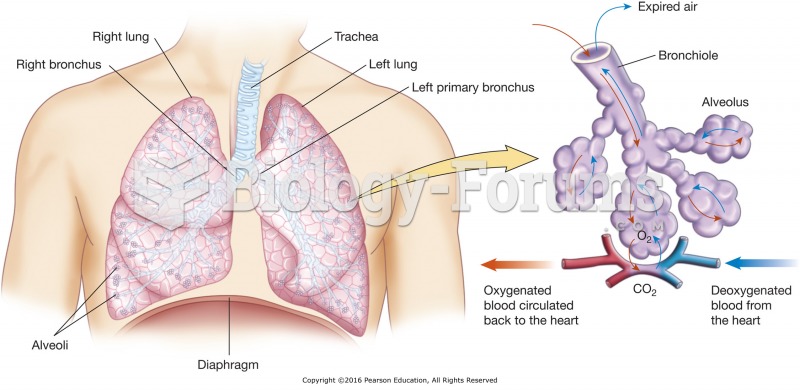
Your heart beats over 36 million times a year.
Did you know?
The tallest man ever known was Robert Wadlow, an American, who reached the height of 8 feet 11 inches. He died at age 26 years from an infection caused by the immense weight of his body (491 pounds) and the stress on his leg bones and muscles.
Did you know?
As of mid-2016, 18.2 million people were receiving advanced retroviral therapy (ART) worldwide. This represents between 43–50% of the 34–39.8 million people living with HIV.
Did you know?
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.