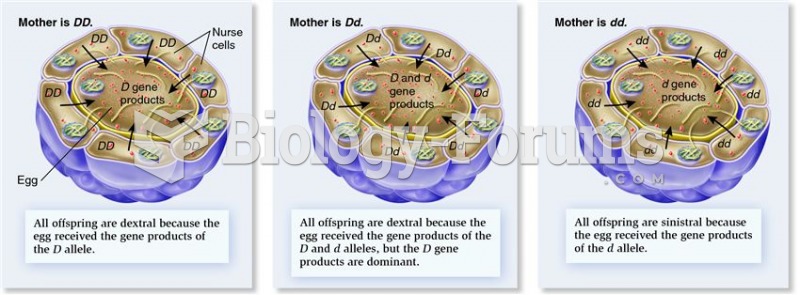
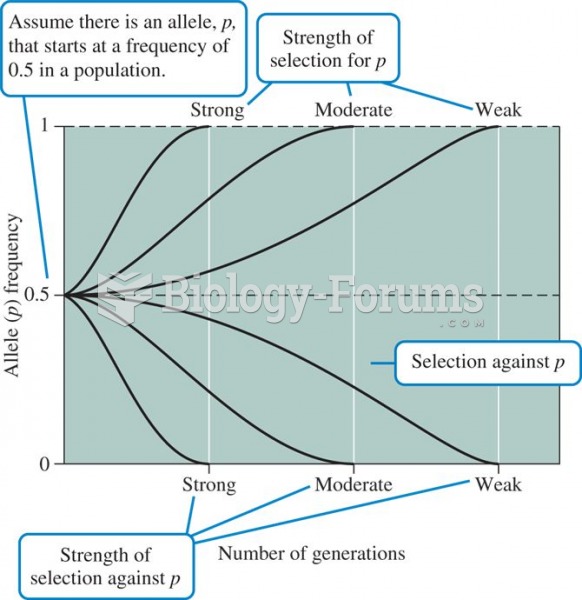
|
|
|
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
When intravenous medications are involved in adverse drug events, their harmful effects may occur more rapidly, and be more severe than errors with oral medications. This is due to the direct administration into the bloodstream.
About 60% of newborn infants in the United States are jaundiced; that is, they look yellow. Kernicterus is a form of brain damage caused by excessive jaundice. When babies begin to be affected by excessive jaundice and begin to have brain damage, they become excessively lethargic.
In the United States, there is a birth every 8 seconds, according to the U.S. Census Bureau's Population Clock.
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.