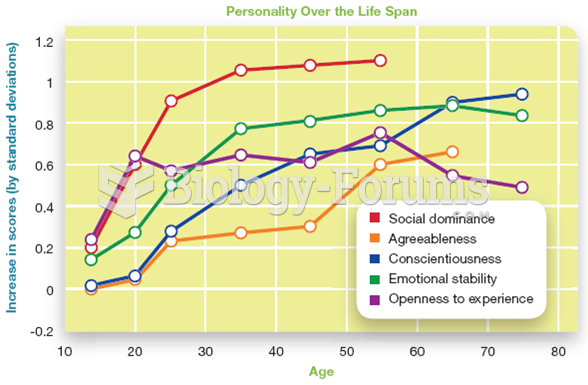
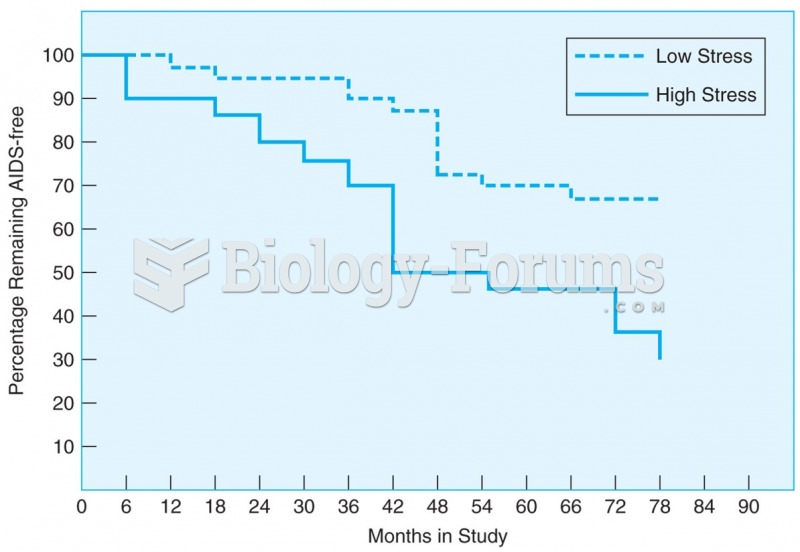
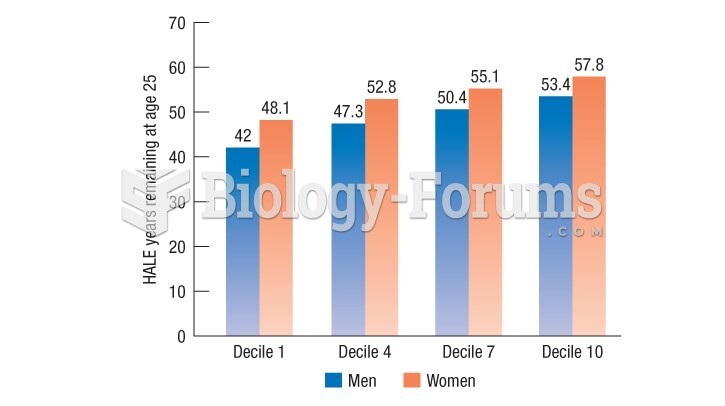
|
|
|
A good example of polar molecules can be understood when trying to make a cake. If water and oil are required, they will not mix together. If you put them into a measuring cup, the oil will rise to the top while the water remains on the bottom.
According to the Migraine Research Foundation, migraines are the third most prevalent illness in the world. Women are most affected (18%), followed by children of both sexes (10%), and men (6%).
The effects of organophosphate poisoning are referred to by using the abbreviations “SLUD” or “SLUDGE,” It stands for: salivation, lacrimation, urination, defecation, GI upset, and emesis.
Giardia is one of the most common intestinal parasites worldwide, and infects up to 20% of the world population, mostly in poorer countries with inadequate sanitation. Infections are most common in children, though chronic Giardia is more common in adults.
In most climates, 8 to 10 glasses of water per day is recommended for adults. The best indicator for adequate fluid intake is frequent, clear urination.