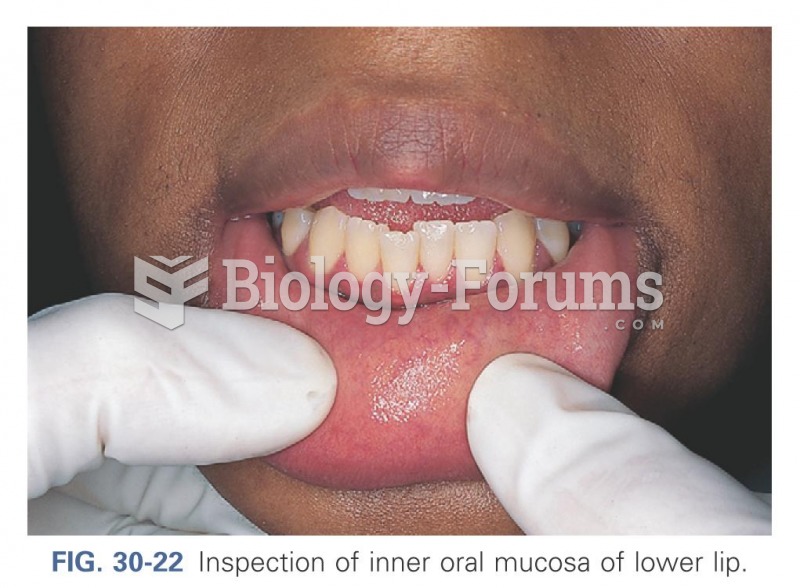
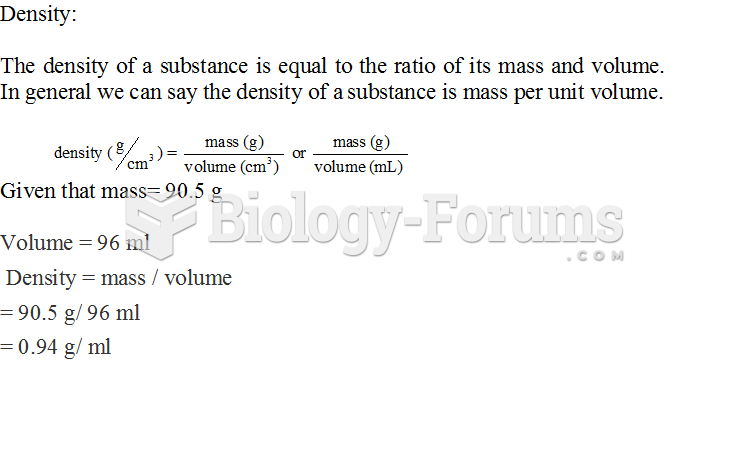
|
|
|
Complications of influenza include: bacterial pneumonia, ear and sinus infections, dehydration, and worsening of chronic conditions such as asthma, congestive heart failure, or diabetes.
The Babylonians wrote numbers in a system that used 60 as the base value rather than the number 10. They did not have a symbol for "zero."
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
The Centers for Disease Control and Prevention (CDC) was originally known as the Communicable Disease Center, which was formed to fight malaria. It was originally headquartered in Atlanta, Georgia, since the Southern states faced the worst threat from malaria.
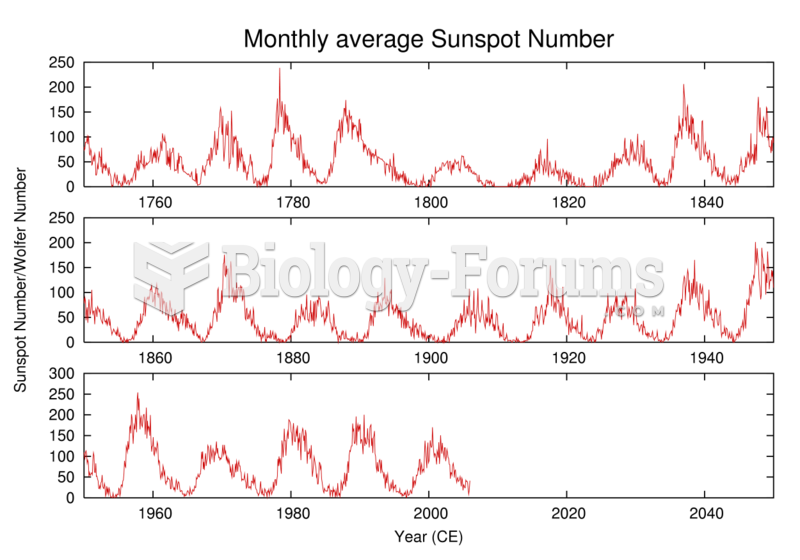 History of the number of observed sunspots during the last 250 years, which shows the ~11-year solar
History of the number of observed sunspots during the last 250 years, which shows the ~11-year solar
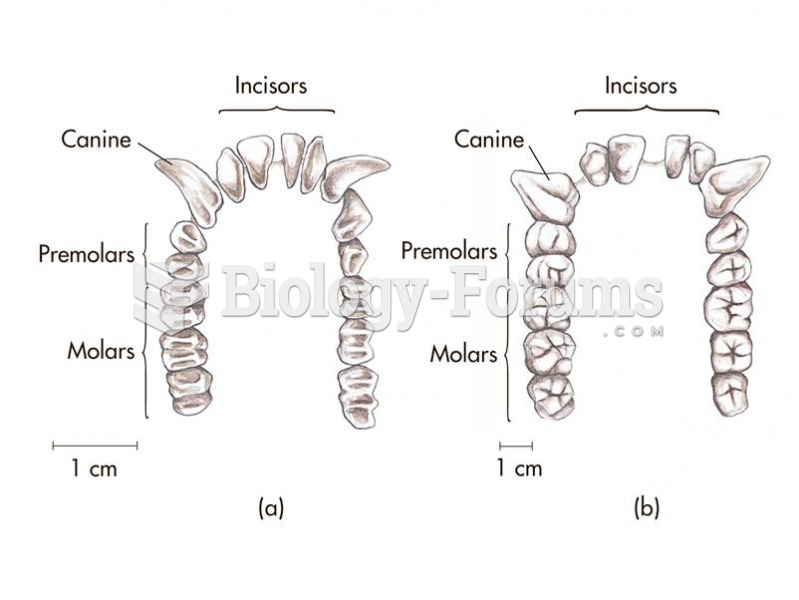 The primate dental formula illustrated for (a) the lower dentition of an Old World monkey and (b) th
The primate dental formula illustrated for (a) the lower dentition of an Old World monkey and (b) th
 Increasing access to care for teen mothers is important because they have a higher rate of pregnancy ...
Increasing access to care for teen mothers is important because they have a higher rate of pregnancy ...