|
|
|
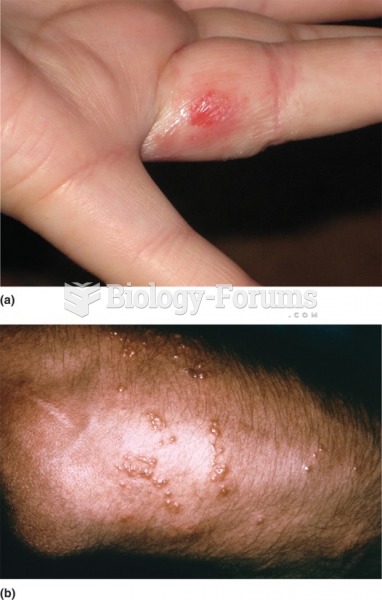
Dogs have been used in studies to detect various cancers in human subjects. They have been trained to sniff breath samples from humans that were collected by having them breathe into special tubes. These people included 55 lung cancer patients, 31 breast cancer patients, and 83 cancer-free patients. The dogs detected 54 of the 55 lung cancer patients as having cancer, detected 28 of the 31 breast cancer patients, and gave only three false-positive results (detecting cancer in people who didn't have it).
The first successful kidney transplant was performed in 1954 and occurred in Boston. A kidney from an identical twin was transplanted into his dying brother's body and was not rejected because it did not appear foreign to his body.
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
As the western states of America were settled, pioneers often had to drink rancid water from ponds and other sources. This often resulted in chronic diarrhea, causing many cases of dehydration and death that could have been avoided if clean water had been available.
Medication errors are more common among seriously ill patients than with those with minor conditions.
 Muskox populations remain in the Arctic all year, though they migrate to higher elevations in the wi
Muskox populations remain in the Arctic all year, though they migrate to higher elevations in the wi
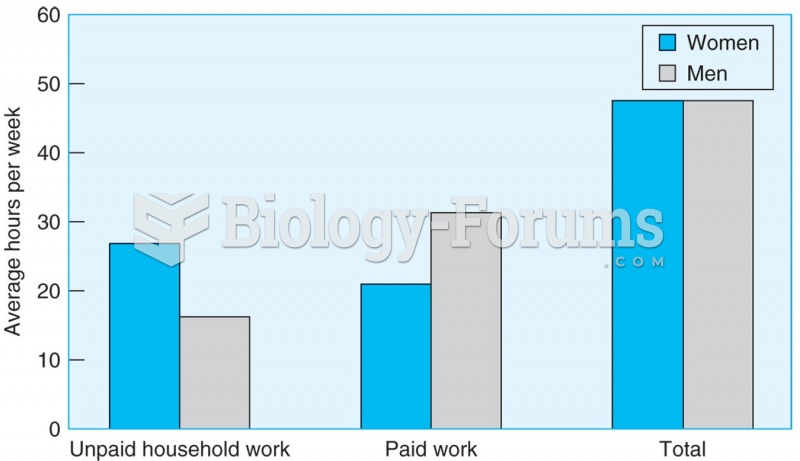 Men and women work the same number of hours a week, but men spend more time on paid work, and women ...
Men and women work the same number of hours a week, but men spend more time on paid work, and women ...
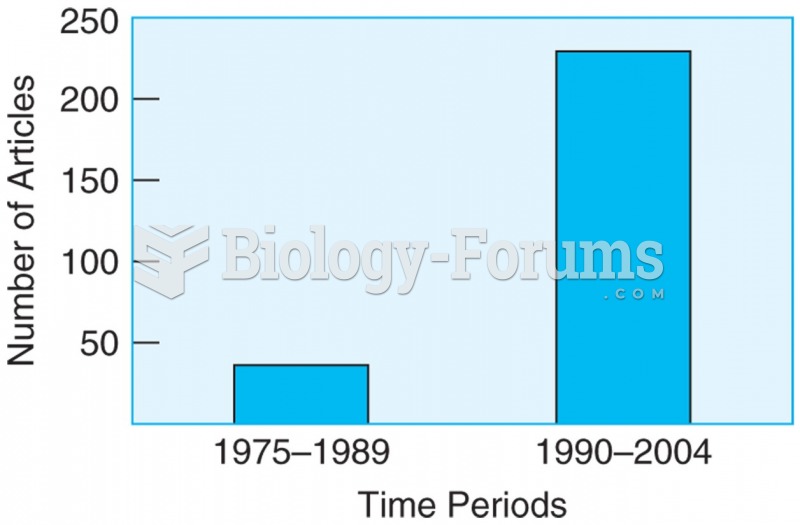 The number of research articles published on the topic of religious practices and spirituality have ...
The number of research articles published on the topic of religious practices and spirituality have ...