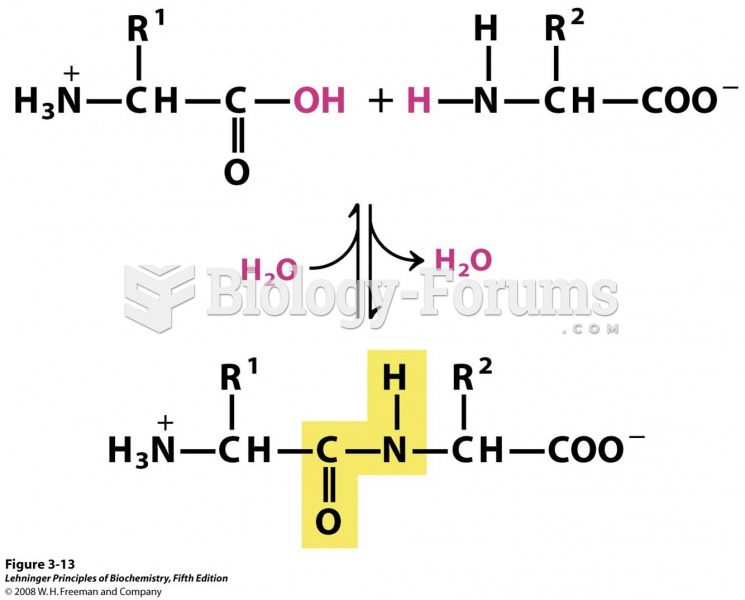
|
|
|
As many as 28% of hospitalized patients requiring mechanical ventilators to help them breathe (for more than 48 hours) will develop ventilator-associated pneumonia. Current therapy involves intravenous antibiotics, but new antibiotics that can be inhaled (and more directly treat the infection) are being developed.
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Pregnant women usually experience a heightened sense of smell beginning late in the first trimester. Some experts call this the body's way of protecting a pregnant woman from foods that are unsafe for the fetus.
The strongest synthetic topical retinoid drug available, tazarotene, is used to treat sun-damaged skin, acne, and psoriasis.
Asthma-like symptoms were first recorded about 3,500 years ago in Egypt. The first manuscript specifically written about asthma was in the year 1190, describing a condition characterized by sudden breathlessness. The treatments listed in this manuscript include chicken soup, herbs, and sexual abstinence.
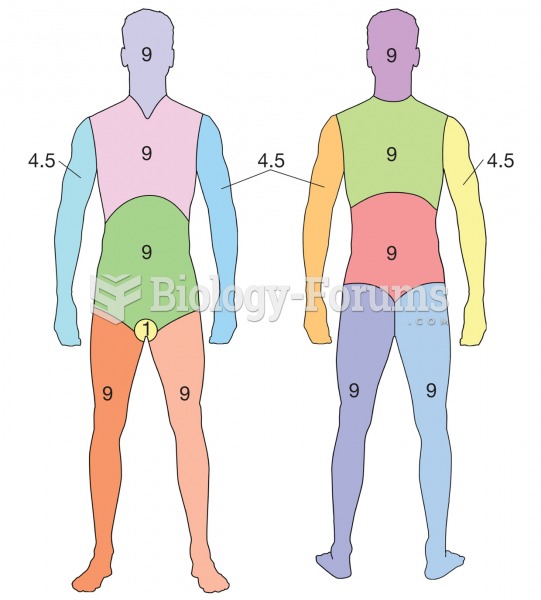 Rule of Nines. A method for determining percentage of body burned. Each different colored section re
Rule of Nines. A method for determining percentage of body burned. Each different colored section re
 Pressure testing the cooling system. A typical hand-operated pressure tester applies pressure equal ...
Pressure testing the cooling system. A typical hand-operated pressure tester applies pressure equal ...