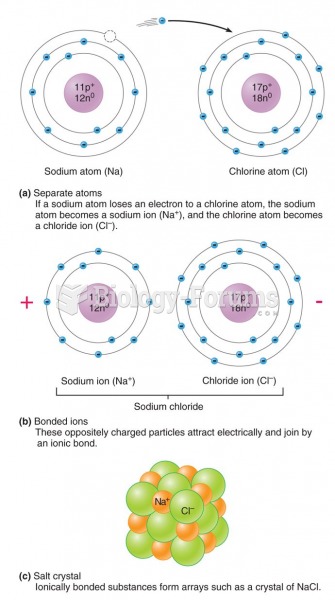
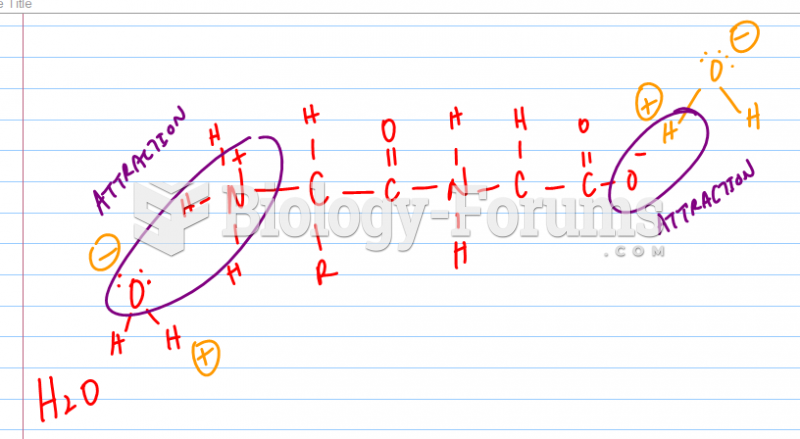
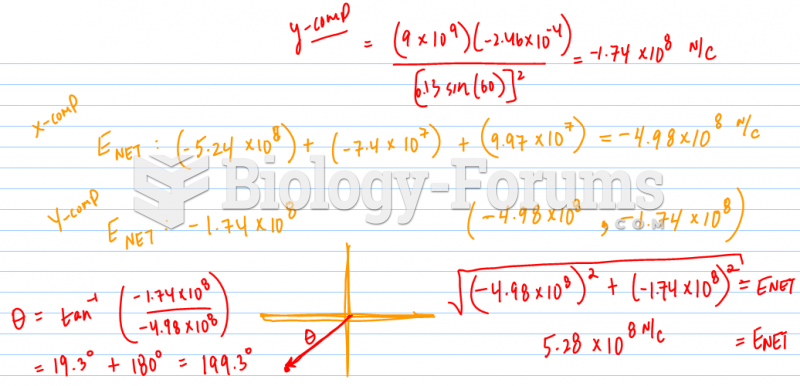
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Did you know?
More than 20 million Americans cite use of marijuana within the past 30 days, according to the National Survey on Drug Use and Health (NSDUH). More than 8 million admit to using it almost every day.