|
|
|
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Intradermal injections are somewhat difficult to correctly administer because the skin layers are so thin that it is easy to accidentally punch through to the deeper subcutaneous layer.
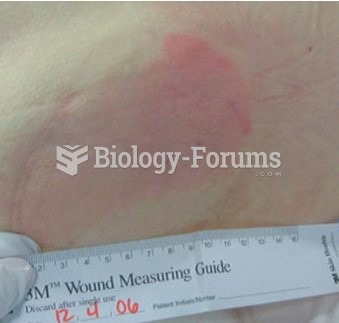
Stevens-Johnson syndrome and Toxic Epidermal Necrolysis syndrome are life-threatening reactions that can result in death. Complications include permanent blindness, dry-eye syndrome, lung damage, photophobia, asthma, chronic obstructive pulmonary disease, permanent loss of nail beds, scarring of mucous membranes, arthritis, and chronic fatigue syndrome. Many patients' pores scar shut, causing them to retain heat.
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.