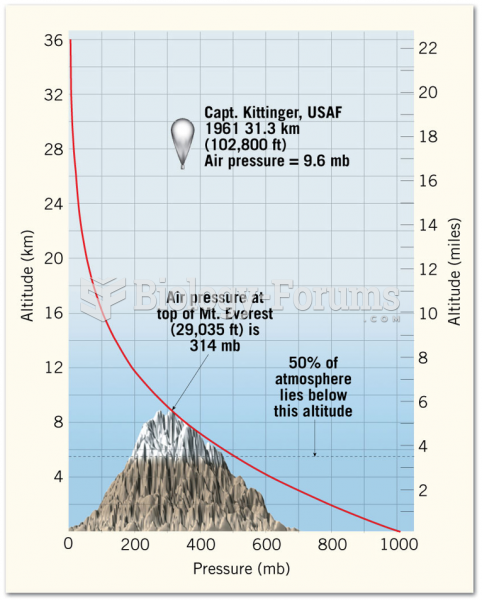
|
|
|
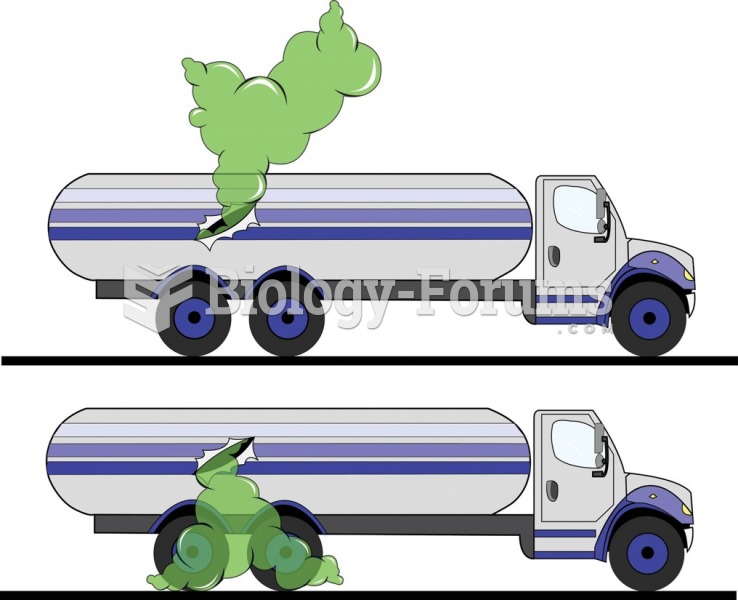
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.
According to the Migraine Research Foundation, migraines are the third most prevalent illness in the world. Women are most affected (18%), followed by children of both sexes (10%), and men (6%).
Medications that are definitely not safe to take when breastfeeding include radioactive drugs, antimetabolites, some cancer (chemotherapy) agents, bromocriptine, ergotamine, methotrexate, and cyclosporine.
The National Institutes of Health have supported research into acupuncture. This has shown that acupuncture significantly reduced pain associated with osteoarthritis of the knee, when used as a complement to conventional therapies.
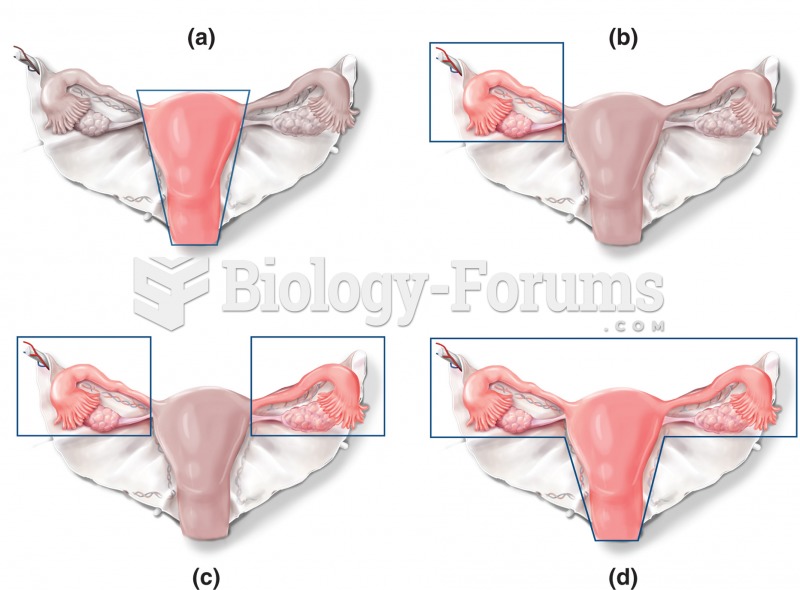 Alternative forms of surgeries involving the uterus, ovaries, and fallopian tubes. The solid lines i
Alternative forms of surgeries involving the uterus, ovaries, and fallopian tubes. The solid lines i
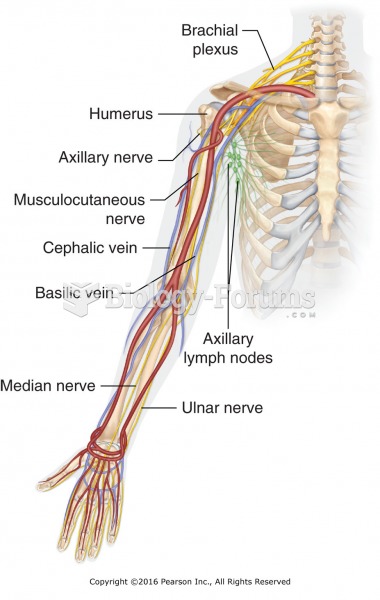 The anterior arm (shoulder, axilla, anticubital, and inner wrist areas). Approach deep pressure in ...
The anterior arm (shoulder, axilla, anticubital, and inner wrist areas). Approach deep pressure in ...