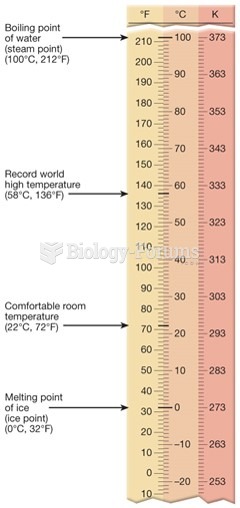
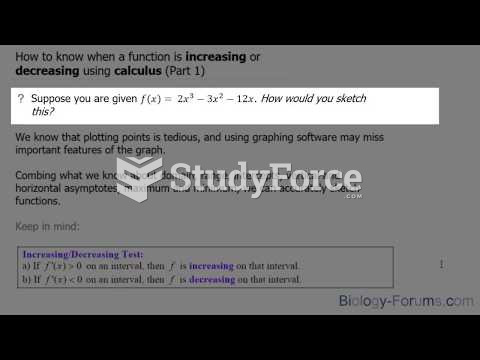
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
Did you know?
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
Did you know?
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
Did you know?
There are actually 60 minerals, 16 vitamins, 12 essential amino acids, and three essential fatty acids that your body needs every day.
Did you know?
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.