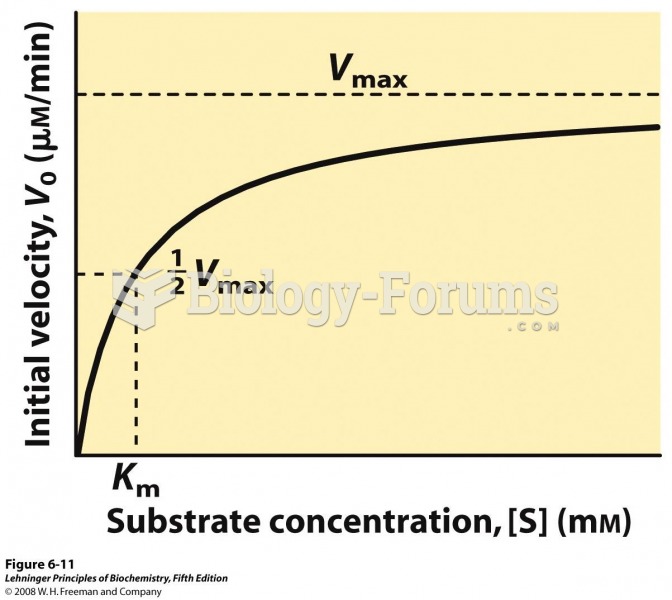
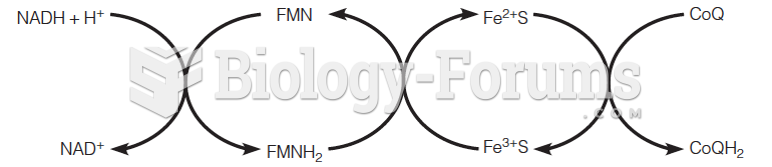
|
|
|
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
Automated pill dispensing systems have alarms to alert patients when the correct dosing time has arrived. Most systems work with many varieties of medications, so patients who are taking a variety of drugs can still be in control of their dose regimen.
In 2010, opiate painkllers, such as morphine, OxyContin®, and Vicodin®, were tied to almost 60% of drug overdose deaths.
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
Common abbreviations that cause medication errors include U (unit), mg (milligram), QD (every day), SC (subcutaneous), TIW (three times per week), D/C (discharge or discontinue), HS (at bedtime or "hours of sleep"), cc (cubic centimeters), and AU (each ear).