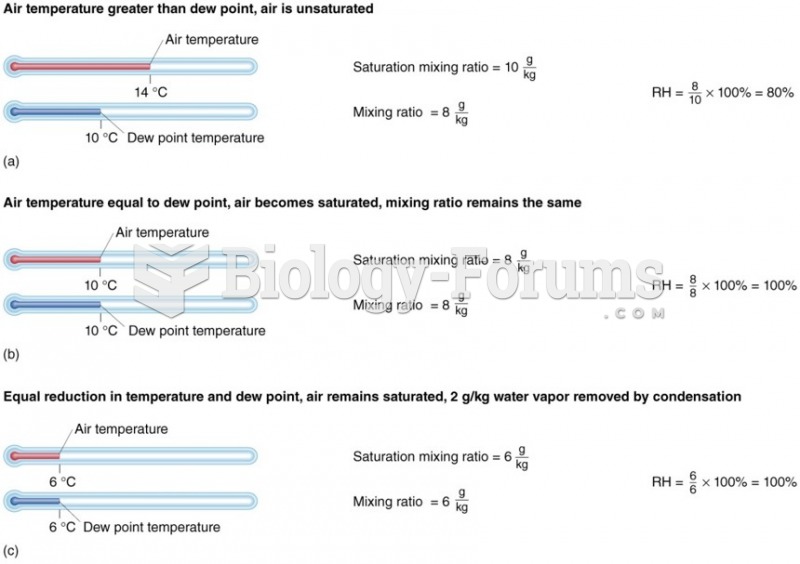
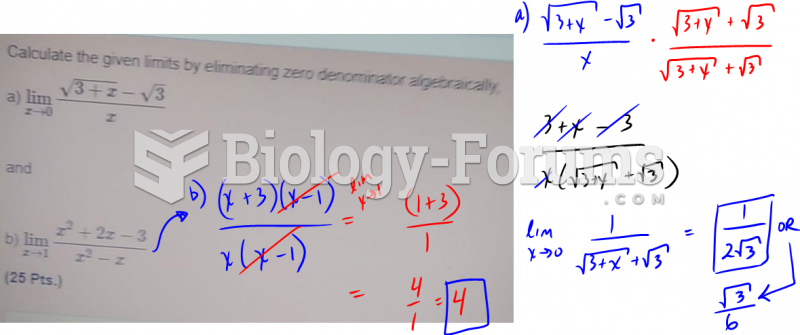
|
|
|
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Dogs have been used in studies to detect various cancers in human subjects. They have been trained to sniff breath samples from humans that were collected by having them breathe into special tubes. These people included 55 lung cancer patients, 31 breast cancer patients, and 83 cancer-free patients. The dogs detected 54 of the 55 lung cancer patients as having cancer, detected 28 of the 31 breast cancer patients, and gave only three false-positive results (detecting cancer in people who didn't have it).
The most common childhood diseases include croup, chickenpox, ear infections, flu, pneumonia, ringworm, respiratory syncytial virus, scabies, head lice, and asthma.
Vaccines prevent between 2.5 and 4 million deaths every year.
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.