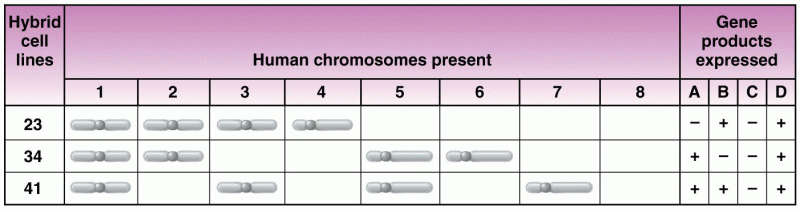
|
|
|
The most common childhood diseases include croup, chickenpox, ear infections, flu, pneumonia, ringworm, respiratory syncytial virus, scabies, head lice, and asthma.
Approximately 70% of expectant mothers report experiencing some symptoms of morning sickness during the first trimester of pregnancy.
Excessive alcohol use costs the country approximately $235 billion every year.
People with alcoholism are at a much greater risk of malnutrition than are other people and usually exhibit low levels of most vitamins (especially folic acid). This is because alcohol often takes the place of 50% of their daily intake of calories, with little nutritional value contained in it.
Nitroglycerin is used to alleviate various heart-related conditions, and it is also the chief component of dynamite (but mixed in a solid clay base to stabilize it).
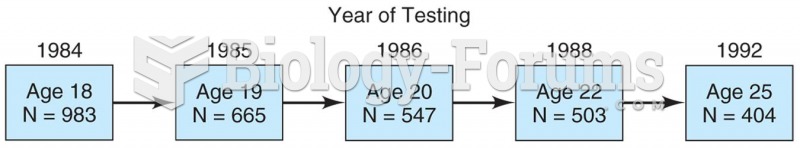 Model of a longitudinal study in which 983 students were surveyed in 1984 and then again in 1985, 19
Model of a longitudinal study in which 983 students were surveyed in 1984 and then again in 1985, 19
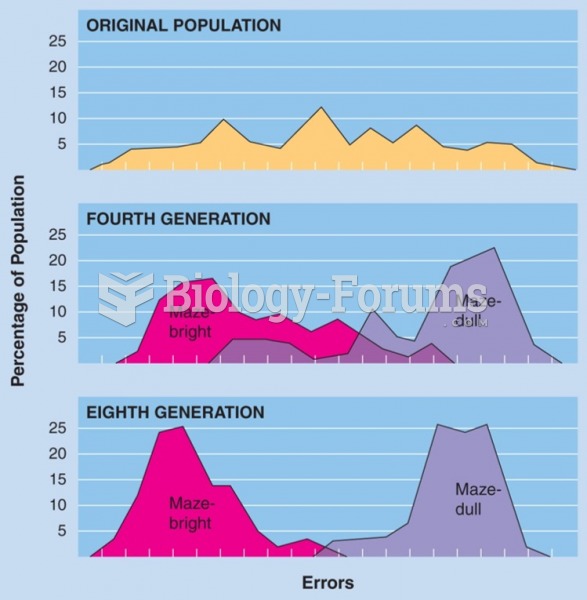 Selective breeding of maze-bright and maze-dull strains of rats by Tryon (1934). (Data from Cooper, ...
Selective breeding of maze-bright and maze-dull strains of rats by Tryon (1934). (Data from Cooper, ...