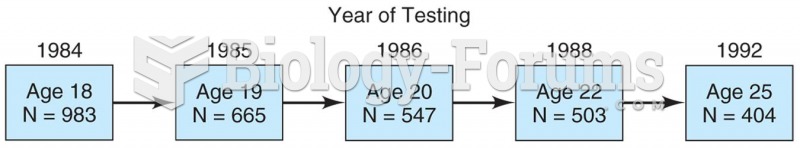
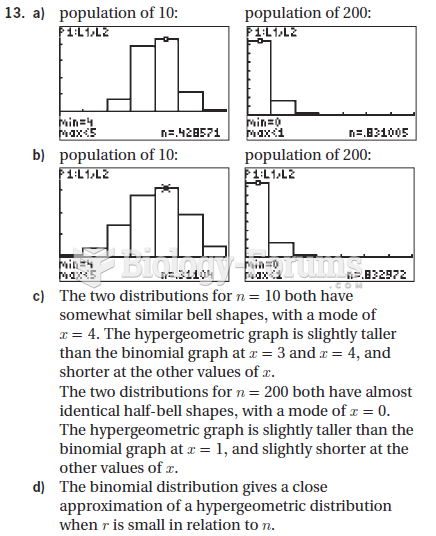
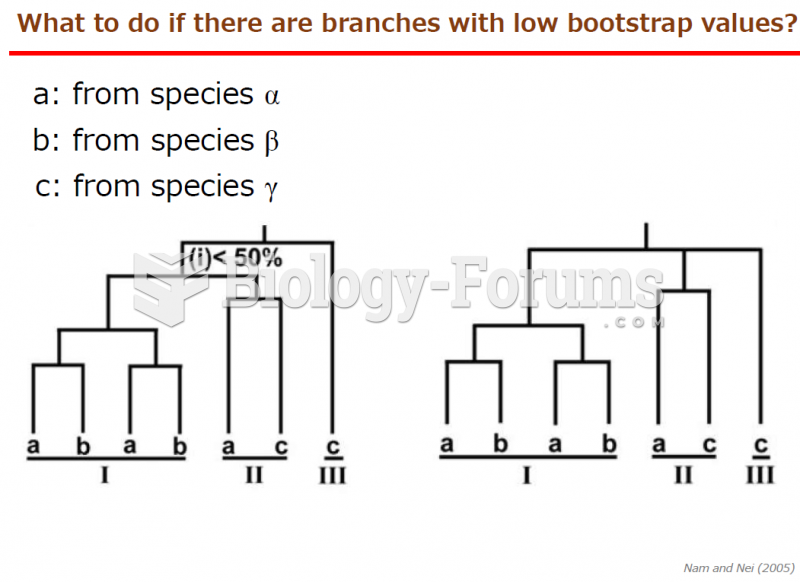
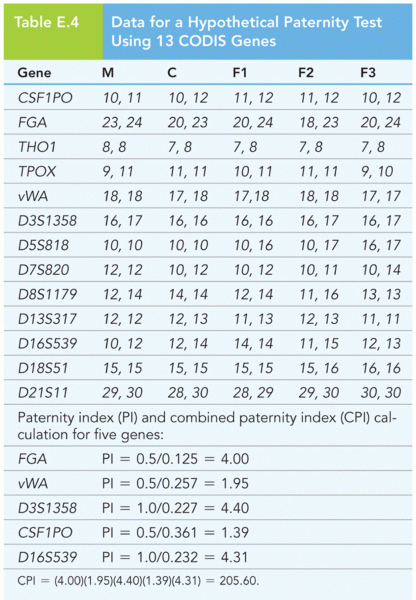
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
Did you know?
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Did you know?
Aspirin may benefit 11 different cancers, including those of the colon, pancreas, lungs, prostate, breasts, and leukemia.
Did you know?
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.
Did you know?
Interferon was scarce and expensive until 1980, when the interferon gene was inserted into bacteria using recombinant DNA technology, allowing for mass cultivation and purification from bacterial cultures.
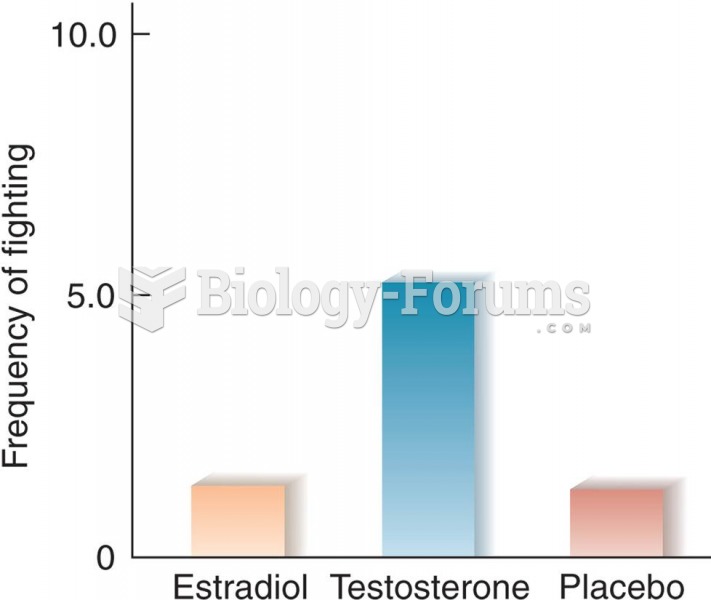 Effects of Estradiol and Testosterone on Interfemale Aggression in Rats (Based on data from van de P
Effects of Estradiol and Testosterone on Interfemale Aggression in Rats (Based on data from van de P
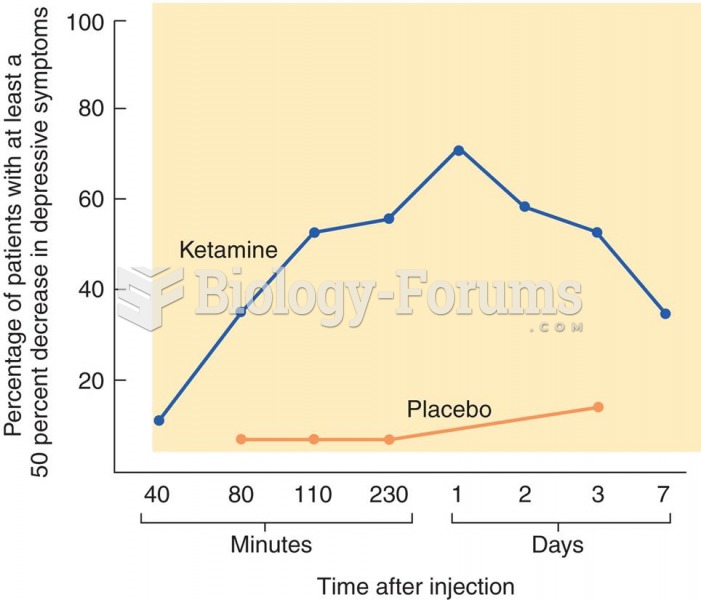 Treatment of Depression with Ketamine The graph shows the effects of ketamine on symptoms of depress
Treatment of Depression with Ketamine The graph shows the effects of ketamine on symptoms of depress