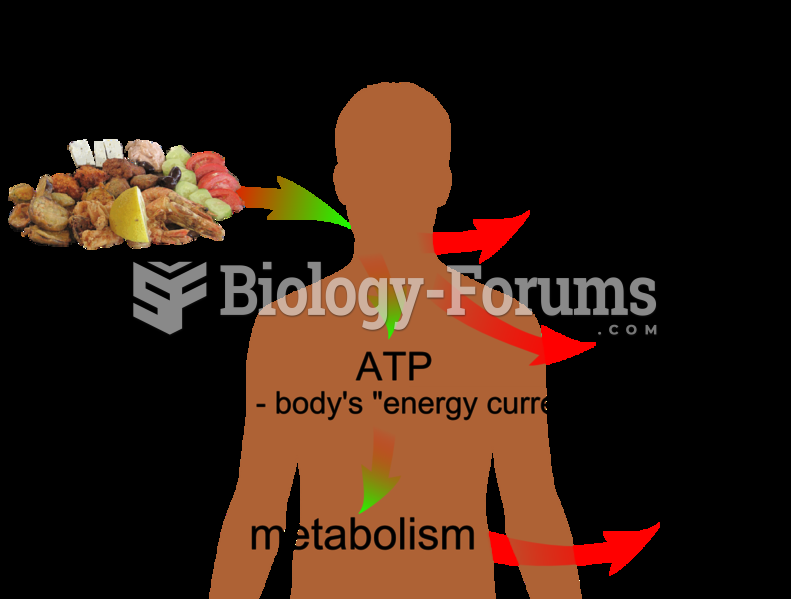
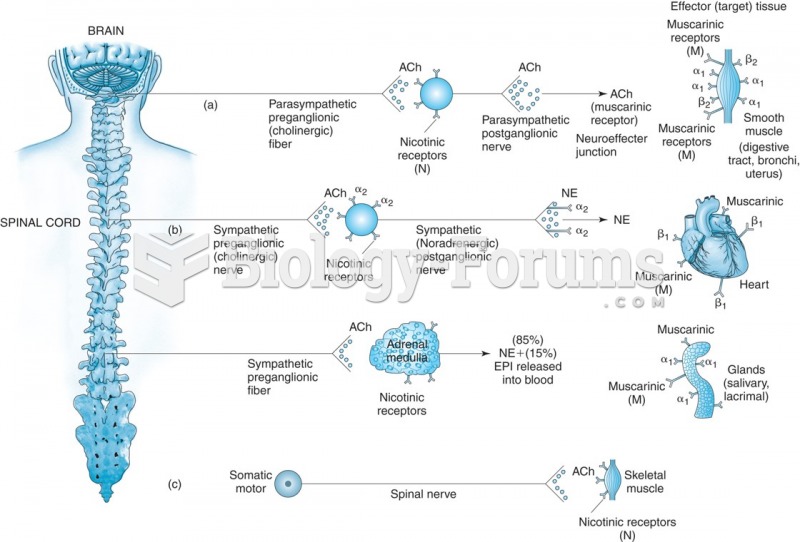
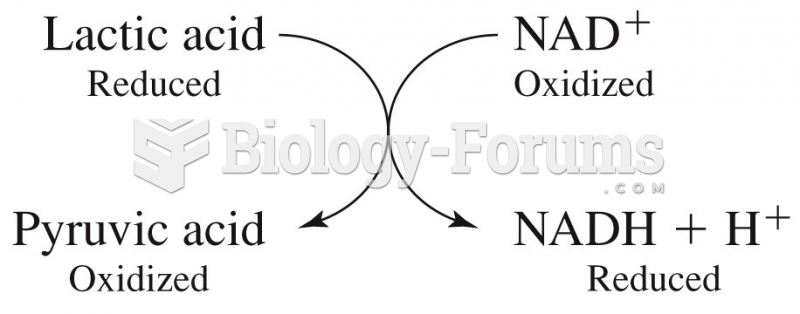
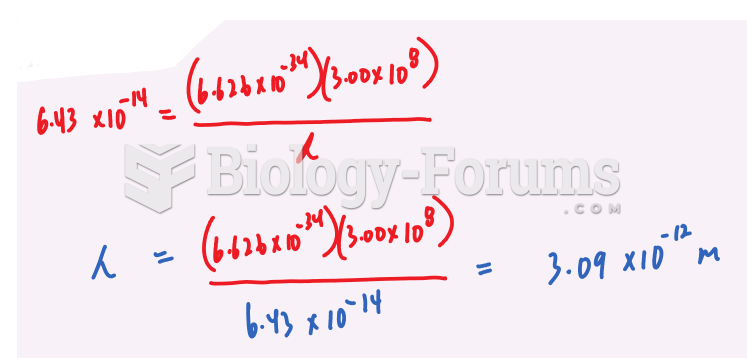
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The average person is easily confused by the terms pharmaceutics and pharmacology, thinking they are one and the same. Whereas pharmaceutics is the science of preparing and dispensing drugs (otherwise known as the science of pharmacy), pharmacology is the study of medications.
Did you know?
The human body produces and destroys 15 million blood cells every second.
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.