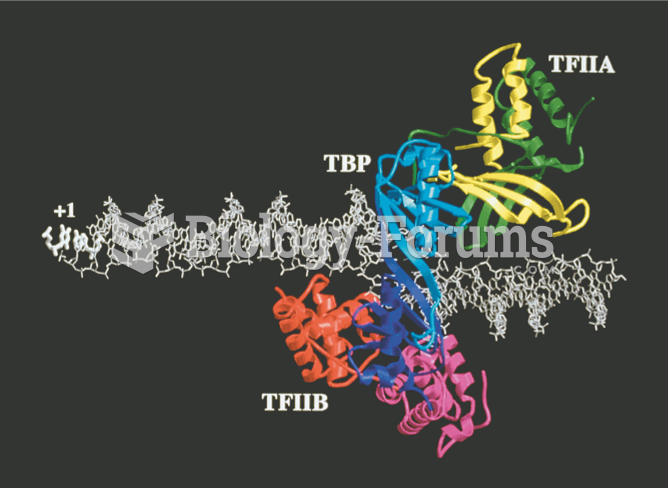
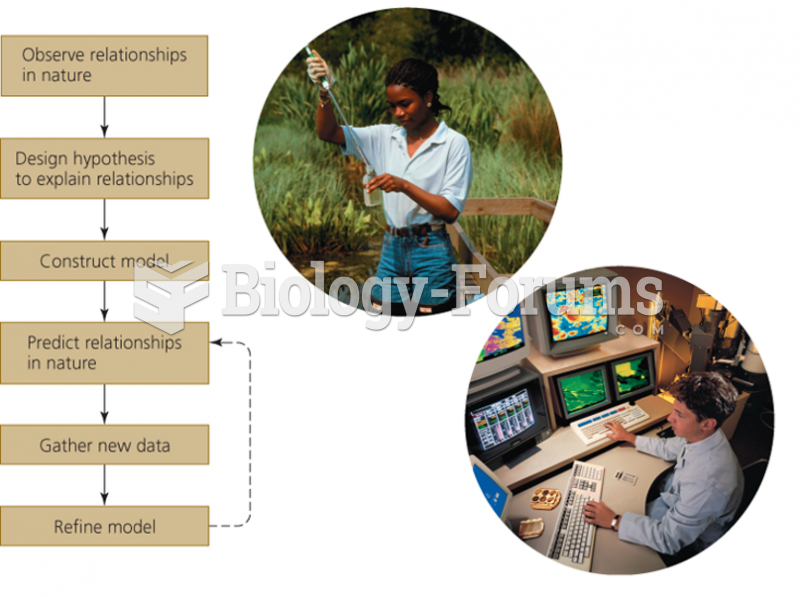
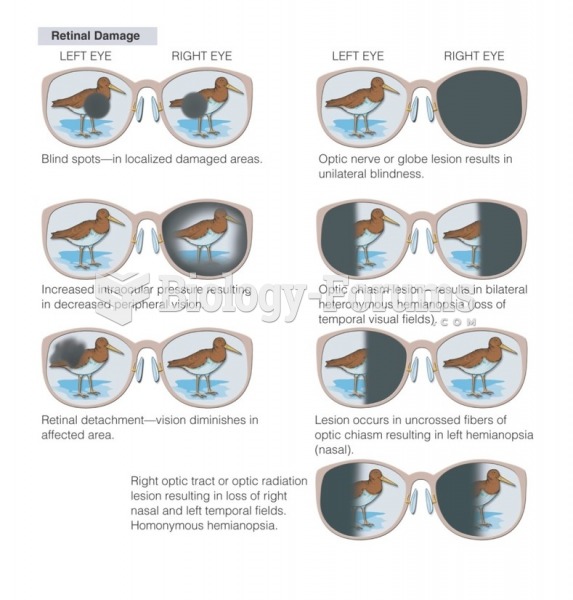
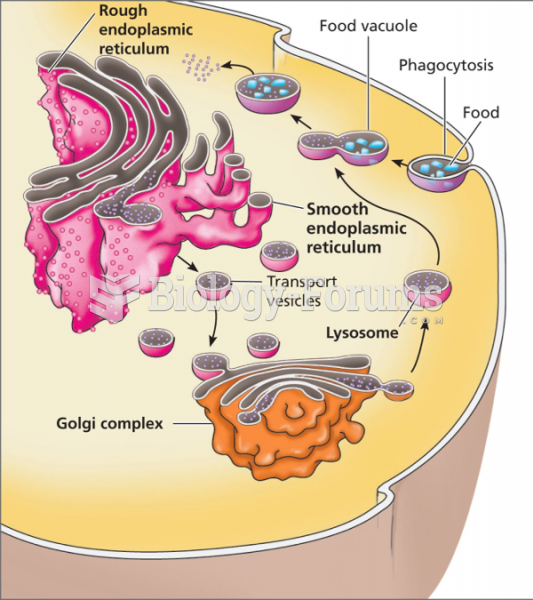
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
Did you know?
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.
Did you know?
The human body's pharmacokinetics are quite varied. Our hair holds onto drugs longer than our urine, blood, or saliva. For example, alcohol can be detected in the hair for up to 90 days after it was consumed. The same is true for marijuana, cocaine, ecstasy, heroin, methamphetamine, and nicotine.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.