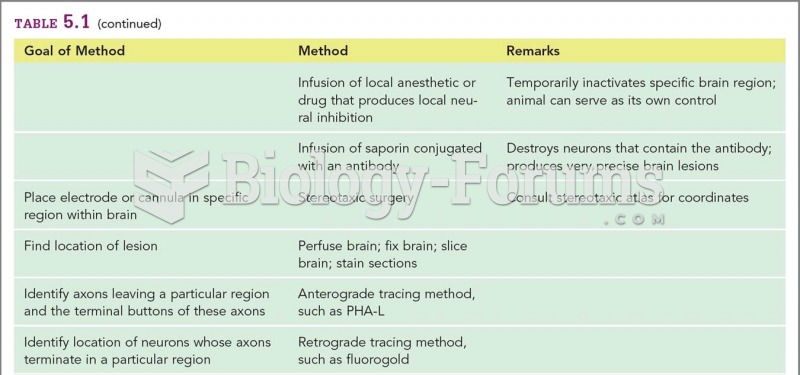
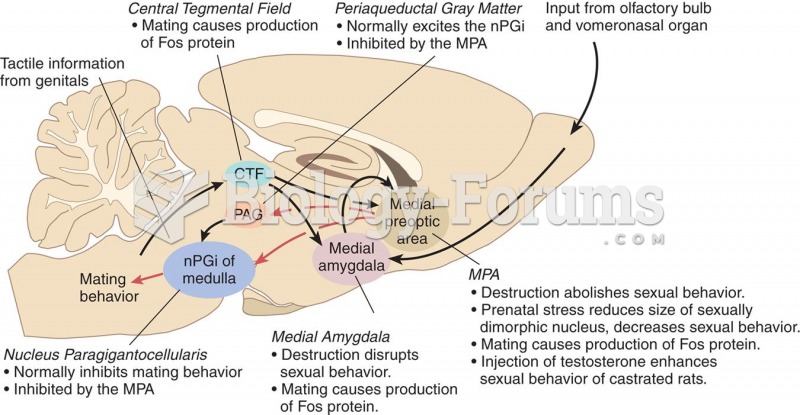
|
|
|
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
People about to have surgery must tell their health care providers about all supplements they take.
Astigmatism is the most common vision problem. It may accompany nearsightedness or farsightedness. It is usually caused by an irregularly shaped cornea, but sometimes it is the result of an irregularly shaped lens. Either type can be corrected by eyeglasses, contact lenses, or refractive surgery.
In the United States, there is a birth every 8 seconds, according to the U.S. Census Bureau's Population Clock.
Prostaglandins were first isolated from human semen in Sweden in the 1930s. They were so named because the researcher thought that they came from the prostate gland. In fact, prostaglandins exist and are synthesized in almost every cell of the body.