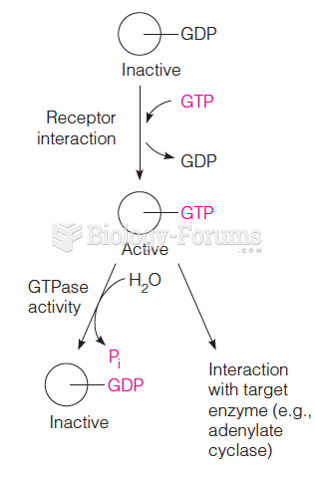
|
|
|
Approximately one in four people diagnosed with diabetes will develop foot problems. Of these, about one-third will require lower extremity amputation.
Fewer than 10% of babies are born on their exact due dates, 50% are born within 1 week of the due date, and 90% are born within 2 weeks of the date.
The U.S. Pharmacopeia Medication Errors Reporting Program states that approximately 50% of all medication errors involve insulin.
Though “Krazy Glue” or “Super Glue” has the ability to seal small wounds, it is not recommended for this purpose since it contains many substances that should not enter the body through the skin, and may be harmful.
Patients who have undergone chemotherapy for the treatment of cancer often complain of a lack of mental focus; memory loss; and a general diminution in abilities such as multitasking, attention span, and general mental agility.
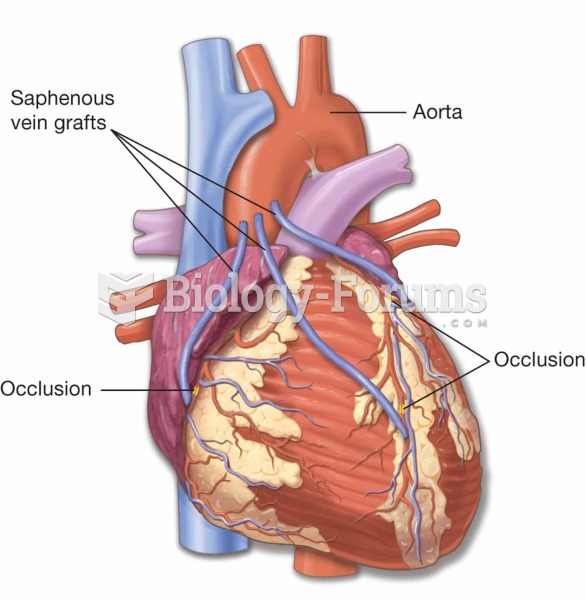 A coronary artery bypass graft (CABG) is a procedure to bypass a blocked coronary artery. The proced
A coronary artery bypass graft (CABG) is a procedure to bypass a blocked coronary artery. The proced
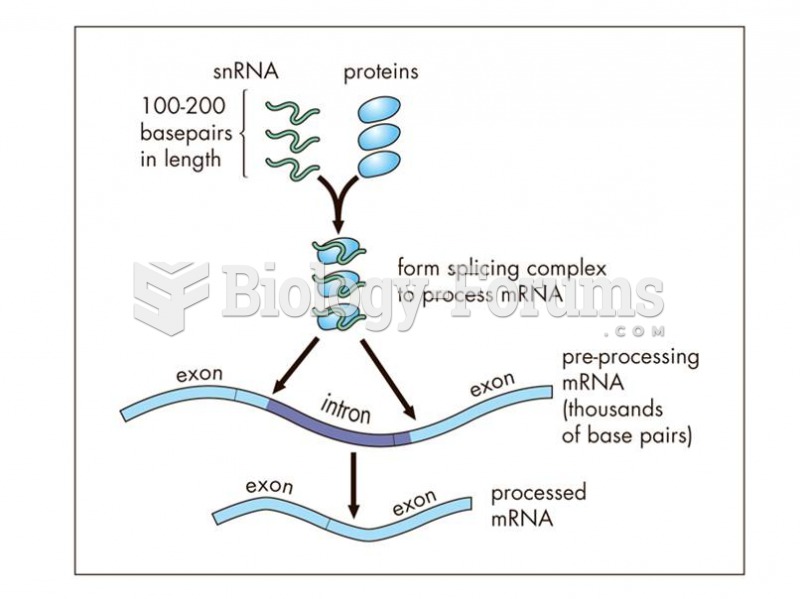 Small nuclear RNAs (snRNA), 100-200 bp in length, form part of the splicing mechanisms to process mR
Small nuclear RNAs (snRNA), 100-200 bp in length, form part of the splicing mechanisms to process mR