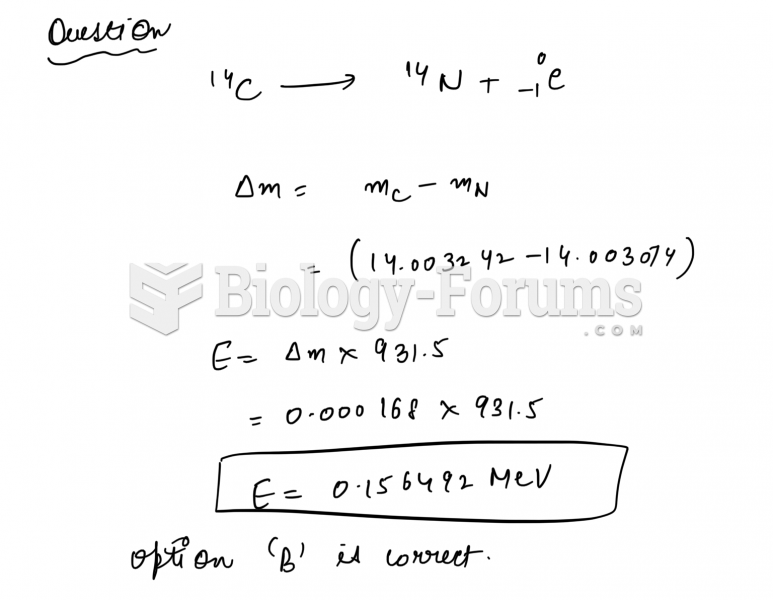
|
|
|
Did you know?
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
Did you know?
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.