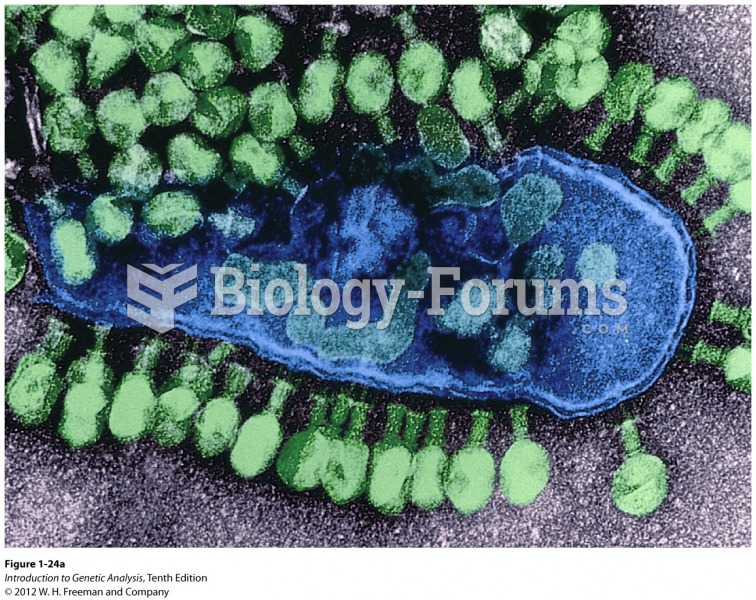
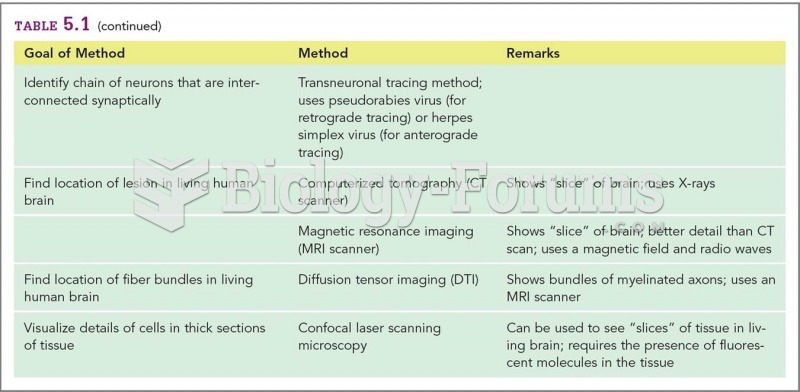
Answer to Question 1
Privacy means that information shared by individual participants will not be shared with others. The most certain way of maintaining privacy is through the provision of anonymity. Anonymity means that there is no personally identifying information in a data set. That is, there is no way that an individual can be identified from the information stored in a researcher's files. When anonymity is possible, it also has a potential benefit in terms of data quality because respondents may be willing to share information more freely.
However, anonymity may not be possible in some research designs. For example, in a longitudinal study, there has to be a way to link records for each measurement occasion at the individual level, necessitating a coding system based on a unique personal identifier.
Alternatively, confidentiality means that the researcher makes a promise that whatever identifying information is shared will be known only by the researcher, unless circumstances dictate exceptions to maintain the well-being of participants. For example, researchers who study sensitive topics such as depression and suicidal thinking may need to maintain a record of the identity of individuals in the event that a red flag of significant distress and likelihood of harm appears in the data. In such cases, research protocols spell out the steps that a researcher will take to provide intervention and support to such a person. The informed consent process should always include clear information about what sort of privacy is promised and what exceptions to confidentiality are necessary.
Privacy of participants receives special protection when medical records are part of the study. The Health Insurance Portability and Accountability Act (HIPAA) of 1996 requires that researchers obtain special permission to utilize data kept in medical records. This may include obtaining permission directly from patients. It may also include obtaining a waiver of permission when it is practically impossible to obtain permission, or a process called de-identification in which all identifying information is removed from the data prior to the researcher being able to access it.
Answer to Question 2
b