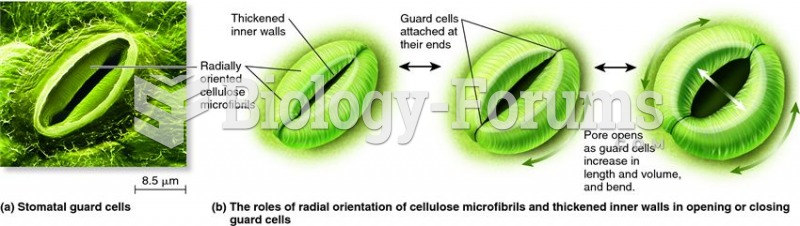
|
|
|
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.
The training of an anesthesiologist typically requires four years of college, 4 years of medical school, 1 year of internship, and 3 years of residency.
Side effects from substance abuse include nausea, dehydration, reduced productivitiy, and dependence. Though these effects usually worsen over time, the constant need for the substance often overcomes rational thinking.
Glaucoma is a leading cause of blindness. As of yet, there is no cure. Everyone is at risk, and there may be no warning signs. It is six to eight times more common in African Americans than in whites. The best and most effective way to detect glaucoma is to receive a dilated eye examination.
Patients who have been on total parenteral nutrition for more than a few days may need to have foods gradually reintroduced to give the digestive tract time to start working again.
 Blocked at every turn by congressional Republicans who hated him, President Obama turned to techniqu
Blocked at every turn by congressional Republicans who hated him, President Obama turned to techniqu
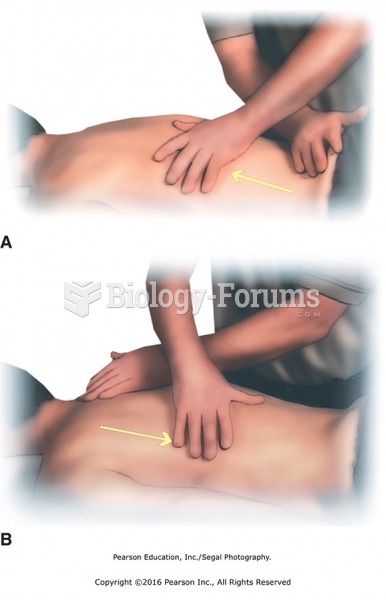
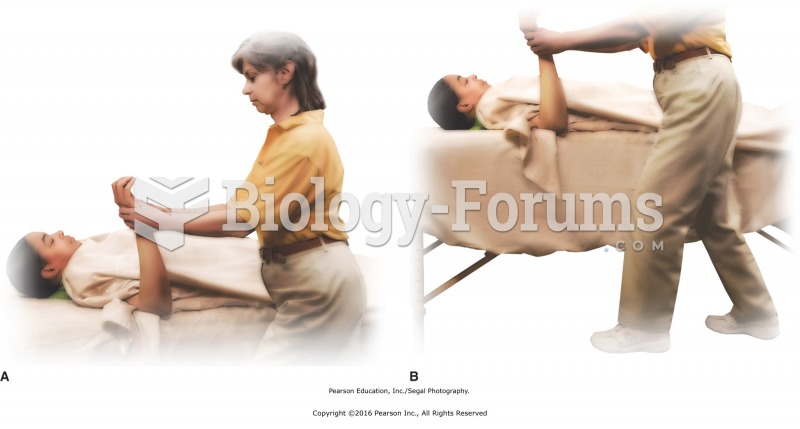 Good body mechanics when facing the head or foot of the table. A. Head and back in alignment. B. ...
Good body mechanics when facing the head or foot of the table. A. Head and back in alignment. B. ...