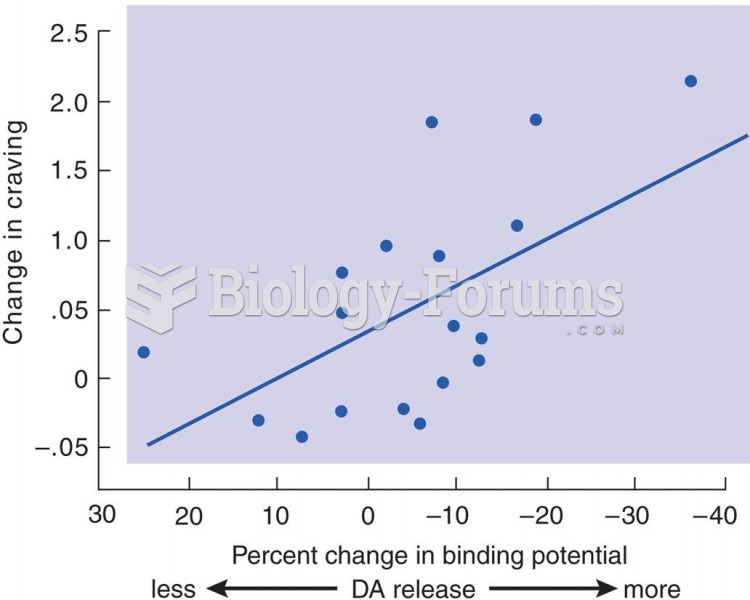
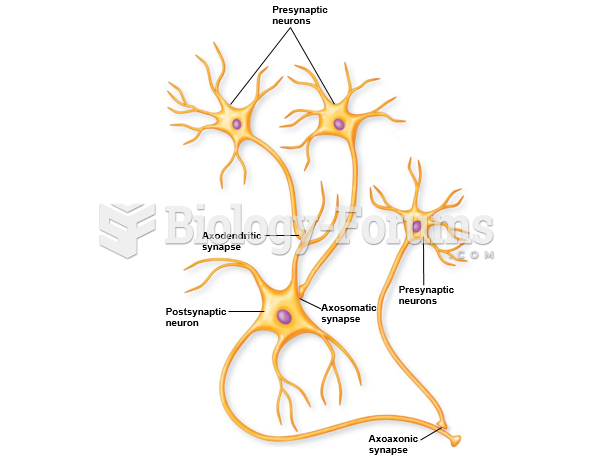
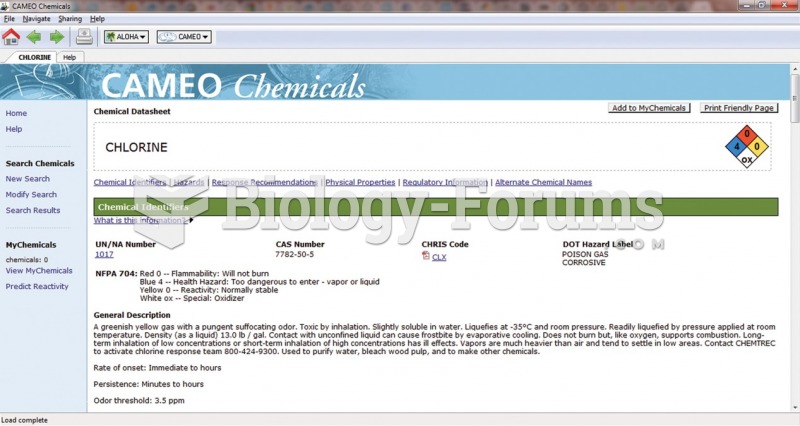
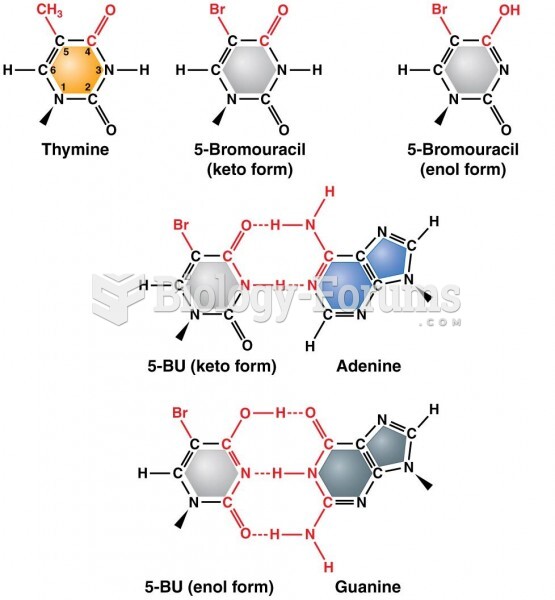
|
|
|
Hypertension is a silent killer because it is deadly and has no significant early symptoms. The danger from hypertension is the extra load on the heart, which can lead to hypertensive heart disease and kidney damage. This occurs without any major symptoms until the high blood pressure becomes extreme. Regular blood pressure checks are an important method of catching hypertension before it can kill you.
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
Pope Sylvester II tried to introduce Arabic numbers into Europe between the years 999 and 1003, but their use did not catch on for a few more centuries, and Roman numerals continued to be the primary number system.
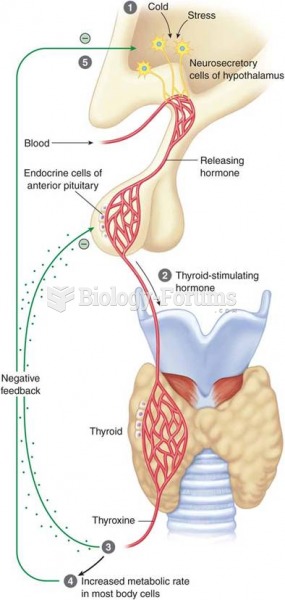 Feedback mechanisms of the thyroid gland: (1) stimulus; (2) release of TSH; (3) release of thyroid h
Feedback mechanisms of the thyroid gland: (1) stimulus; (2) release of TSH; (3) release of thyroid h
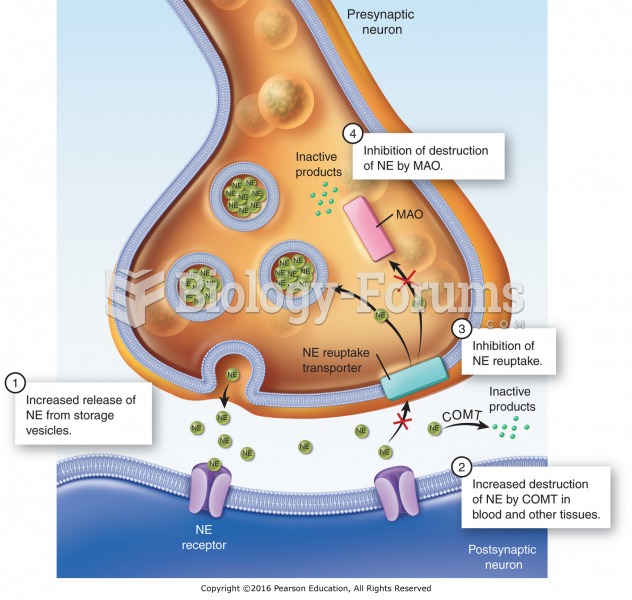 Mechanisms of action of adrenergic agonists: (1) stimulation of the release of NE; (2) increased ...
Mechanisms of action of adrenergic agonists: (1) stimulation of the release of NE; (2) increased ...