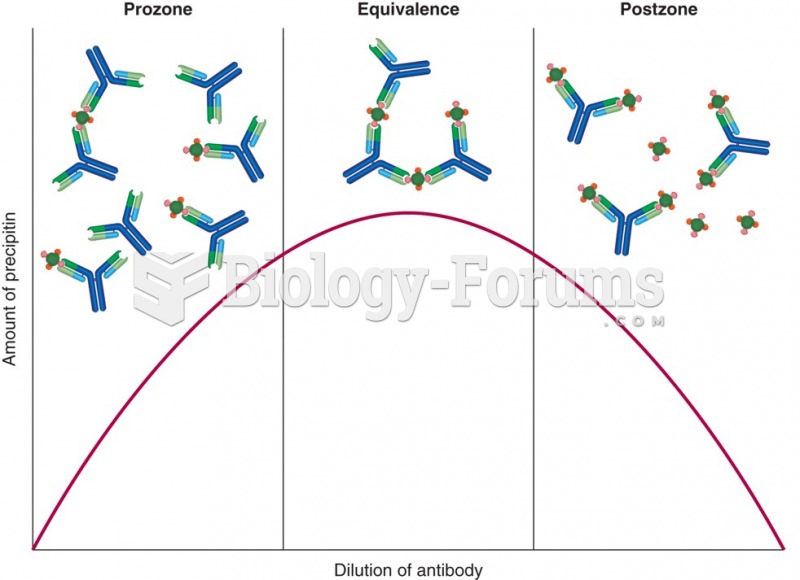
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.
Did you know?
About one in five American adults and teenagers have had a genital herpes infection—and most of them don't know it. People with genital herpes have at least twice the risk of becoming infected with HIV if exposed to it than those people who do not have genital herpes.
Did you know?
The average adult has about 21 square feet of skin.
Did you know?
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
Did you know?
It is difficult to obtain enough calcium without consuming milk or other dairy foods.