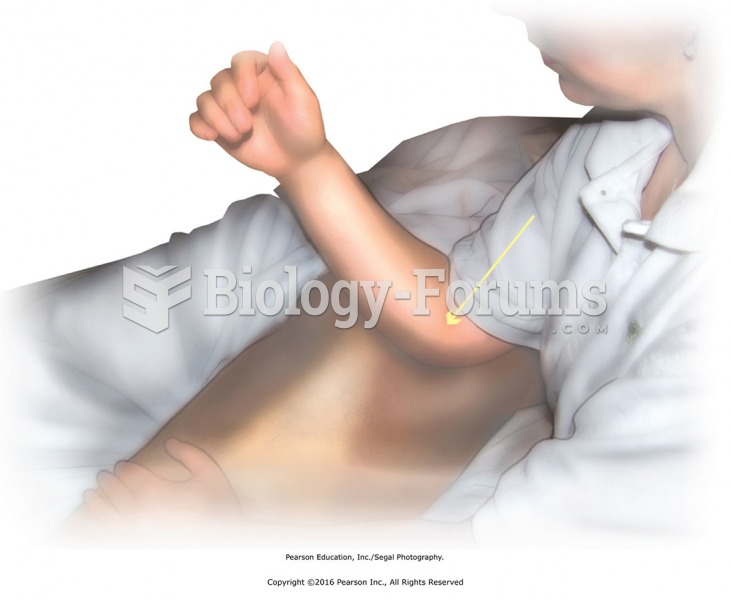
|
|
|
Warfarin was developed as a consequence of the study of a strange bleeding disorder that suddenly occurred in cattle on the northern prairies of the United States in the early 1900s.
Only 12 hours after an egg cell is fertilized by a sperm cell, the egg cell starts to divide. As it continues to divide, it moves along the fallopian tube toward the uterus at about 1 inch per day.
Not getting enough sleep can greatly weaken the immune system. Lack of sleep makes you more likely to catch a cold, or more difficult to fight off an infection.
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
Limit intake of red meat and dairy products made with whole milk. Choose skim milk, low-fat or fat-free dairy products. Limit fried food. Use healthy oils when cooking.
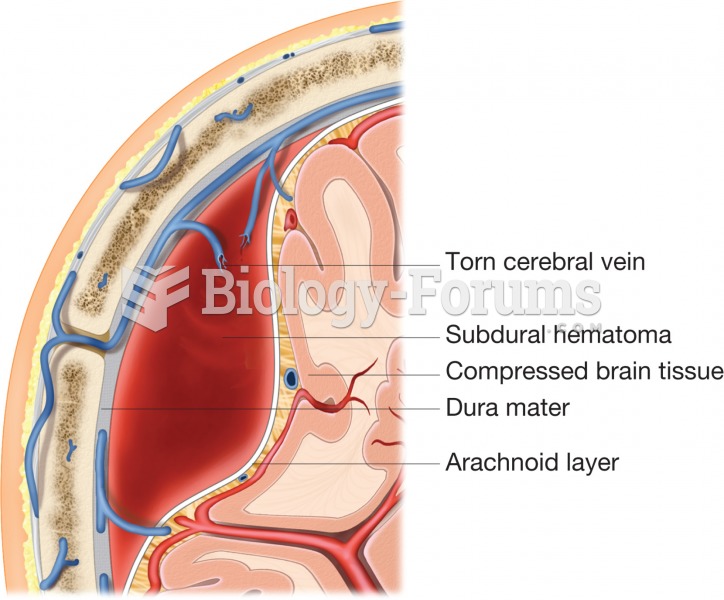 A subdural hematoma. A meningeal vein is ruptured and blood has accumulated in the subdural space, p
A subdural hematoma. A meningeal vein is ruptured and blood has accumulated in the subdural space, p
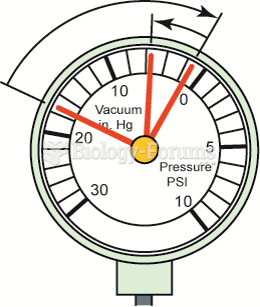 A needle that drops to near zero when the engine is accelerated rapidly and then rises slightly to ...
A needle that drops to near zero when the engine is accelerated rapidly and then rises slightly to ...