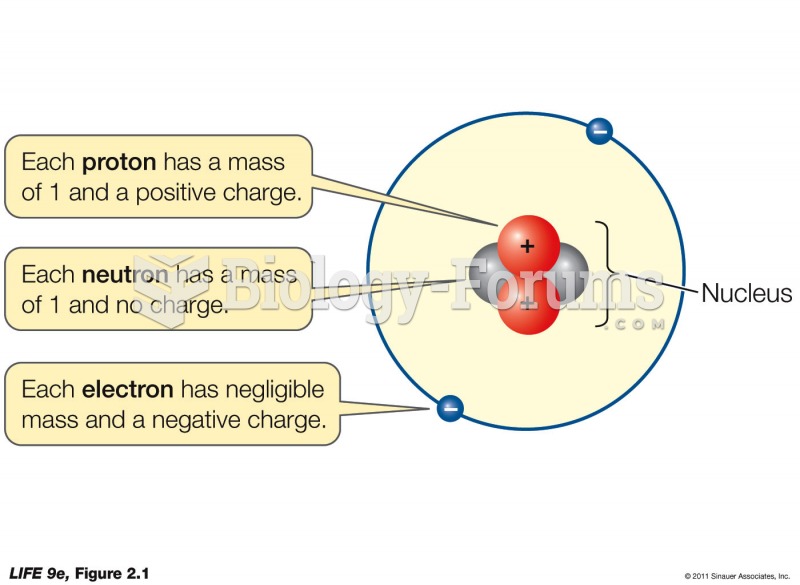
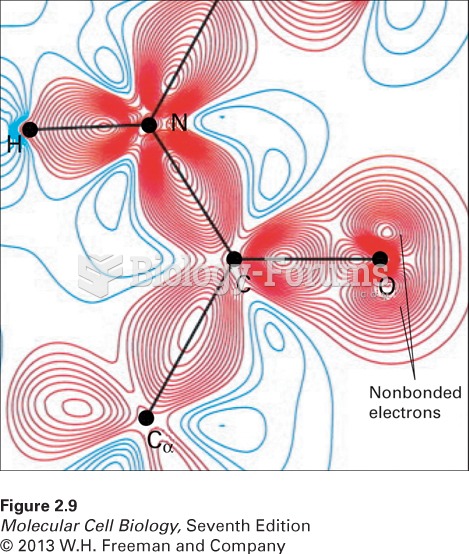
|
|
|
Did you know?
Vaccines prevent between 2.5 and 4 million deaths every year.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Did you know?
Most women experience menopause in their 50s. However, in 1994, an Italian woman gave birth to a baby boy when she was 61 years old.
Did you know?
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Did you know?
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.