|
|
|
A recent study has found that following a diet rich in berries may slow down the aging process of the brain. This diet apparently helps to keep dopamine levels much higher than are seen in normal individuals who do not eat berries as a regular part of their diet as they enter their later years.
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
Nearly all drugs pass into human breast milk. How often a drug is taken influences the amount of drug that will pass into the milk. Medications taken 30 to 60 minutes before breastfeeding are likely to be at peak blood levels when the baby is nursing.
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
The first-known contraceptive was crocodile dung, used in Egypt in 2000 BC. Condoms were also reportedly used, made of animal bladders or intestines.
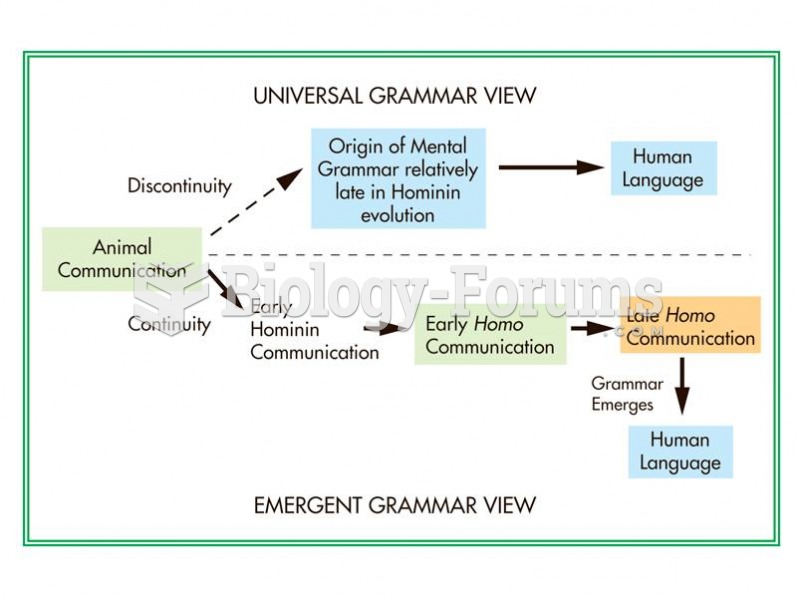 The universal grammar and emergent grammar viewpoint lead to very different scenarios of the evoluti
The universal grammar and emergent grammar viewpoint lead to very different scenarios of the evoluti
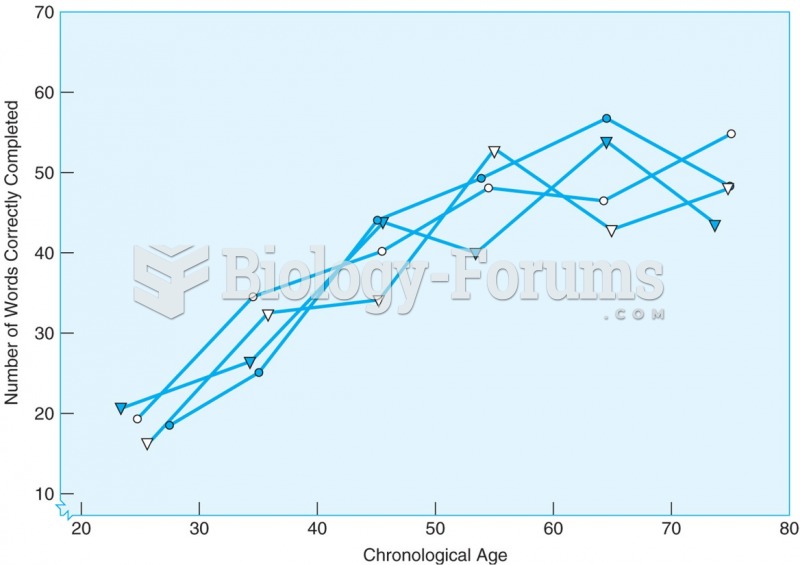 The number of words correctly completed in the New York Times crossword puzzle increases with age ...
The number of words correctly completed in the New York Times crossword puzzle increases with age ...
 Feelings of competence, independence, and relatedness lead to a sense of overall well-being in any ...
Feelings of competence, independence, and relatedness lead to a sense of overall well-being in any ...