|
|
|
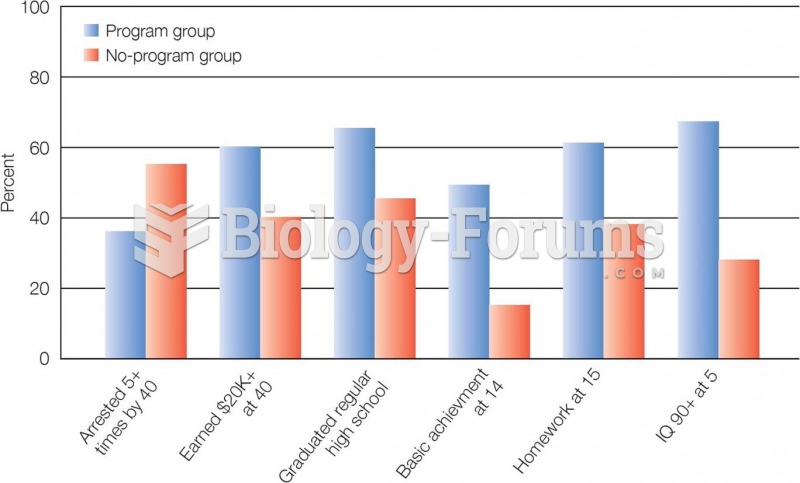
Long-term mental and physical effects from substance abuse include: paranoia, psychosis, immune deficiencies, and organ damage.
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
Amoebae are the simplest type of protozoans, and are characterized by a feeding and dividing trophozoite stage that moves by temporary extensions called pseudopodia or false feet.
As of mid-2016, 18.2 million people were receiving advanced retroviral therapy (ART) worldwide. This represents between 43–50% of the 34–39.8 million people living with HIV.
The modern decimal position system was the invention of the Hindus (around 800 AD), involving the placing of numerals to indicate their value (units, tens, hundreds, and so on).
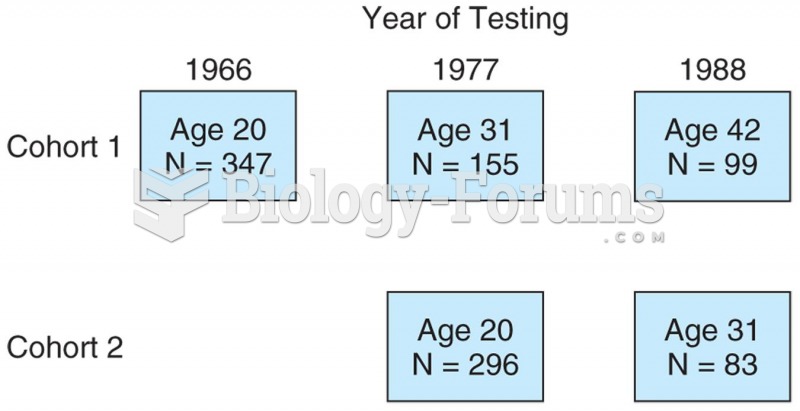 Model of a sequential study in which two cohorts were followed beginning at age 20. One cohort was f
Model of a sequential study in which two cohorts were followed beginning at age 20. One cohort was f
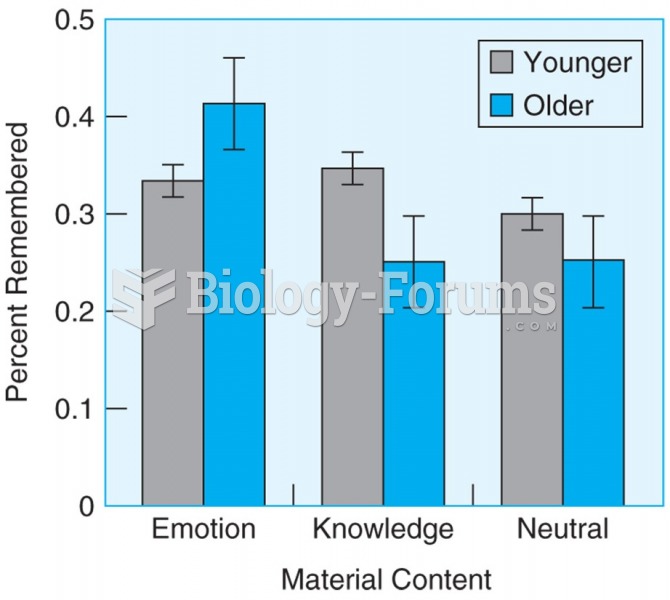 Older participants remember more information than younger participants when material has emotional ...
Older participants remember more information than younger participants when material has emotional ...