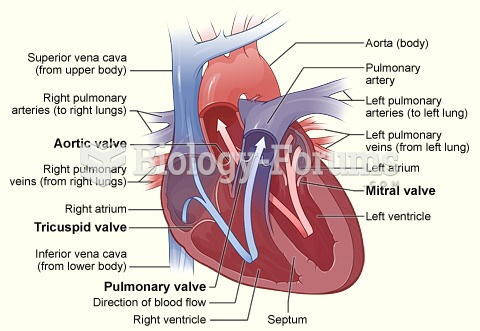
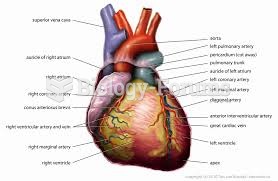
Answer to Question 1
Ans: A
Feedback:
The gold standard for experimental studies is the prospective, double-blind, placebo-control group design, also referred to as clinical trials or therapeutic trials. In double-blind experimental studies, neither the researchers nor the subjects are aware to which group they are randomly assigned. Quasi-experimental and experimental designs are used to examine causality. Many studies, although experimental in design, are not able to either randomize selection of subjects or exert the same degree of control of the study variables that would be found in true experimental studies. Descriptive epidemiologic studies, which are frequently used in public health, are designed to acquire more information about characteristics of health (or disease) as they pertain to person, place, and time. Findings from descriptive epidemiologic studies lead to hypotheses for future research.
Answer to Question 2
Ans: D
Feedback:
Analytical research study designs are on a continuum, ranging from strongest to weakest designs. The research continuum indicates that experimental study designs are the strongest because they control for all factors except that which is under study, with the gold standard for research design being the randomized, control group design. Two analytical designs, the prospective correlational design and the retrospective correlational design, are weaker designs on the continuum. Quasi-experimental study designs are stronger than retrospective studies but weaker than experimental because assignment of subjects into groups is not randomized or the researcher is unable to manipulate the variable under study.