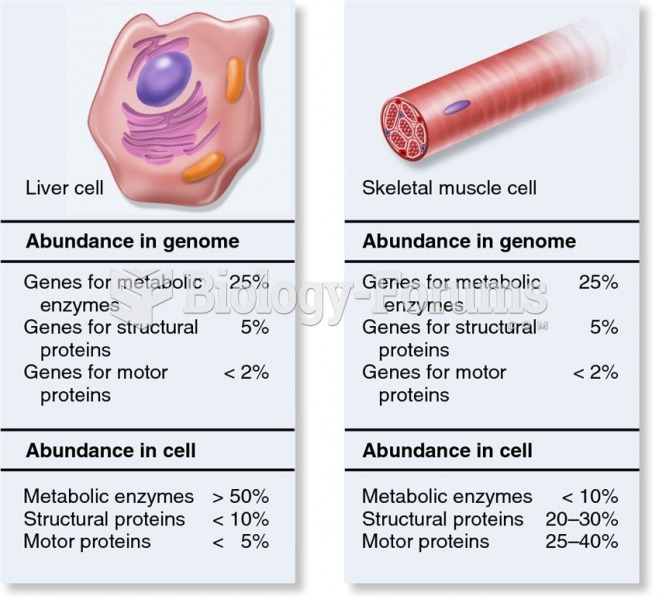
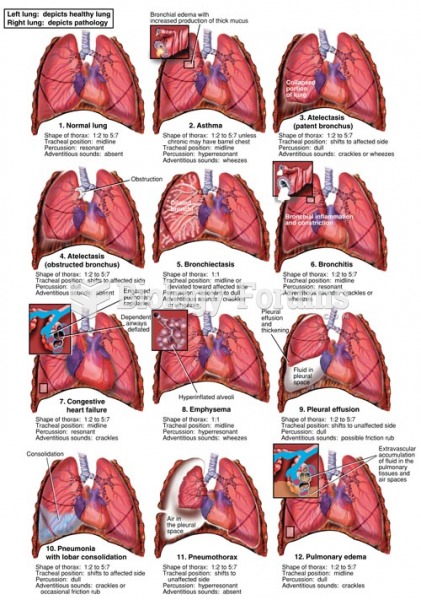
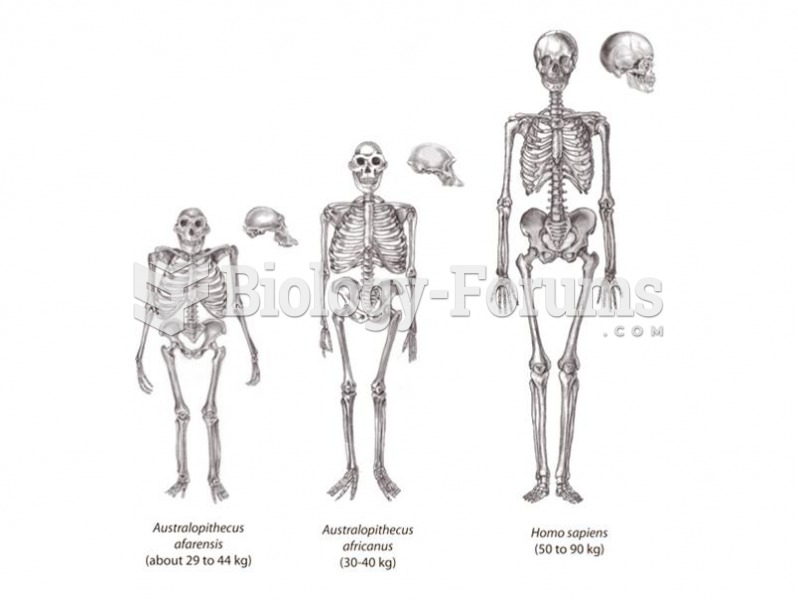
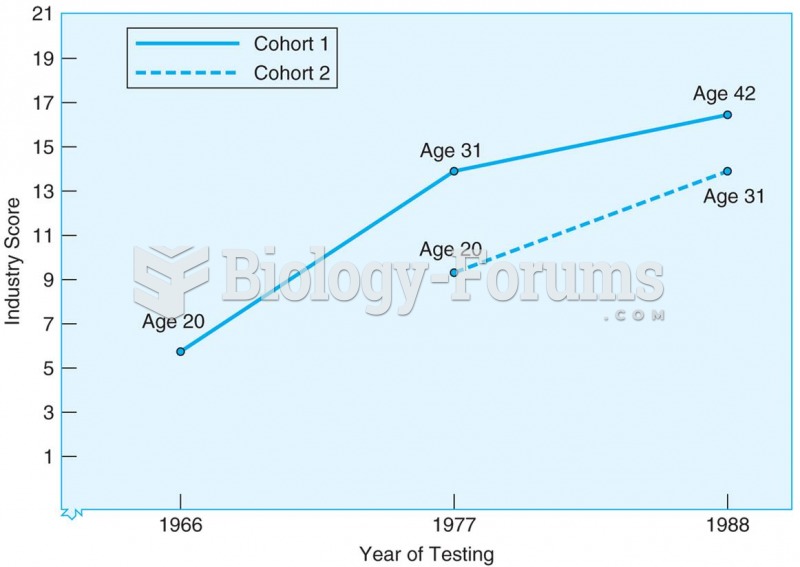
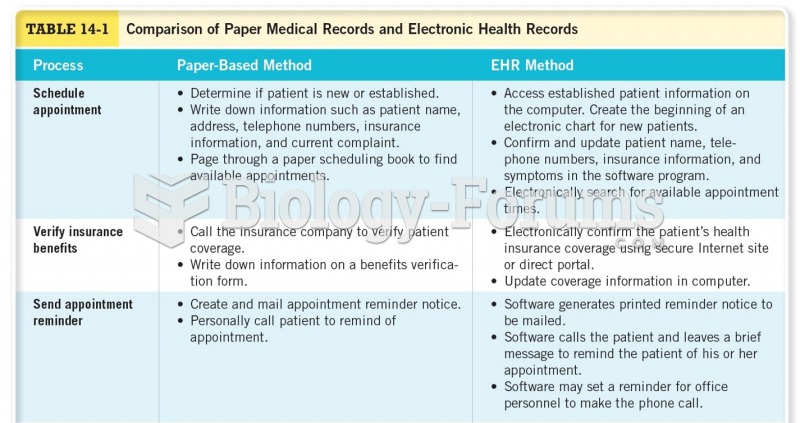
Answer to Question 1
ANS: B
Studies are usually exempt from review if they involve no apparent risks for the research subjects. Studies involving interventions or treatments usually fall into the nonexempt category as do studies of high-risk patients.
The study described in this option involves an invasive procedure and would require complete review
The study described in this option not only involves an invasive procedure, but is using a vulnerable population, both requiring full review.
This study involves a minimal risk, but a vulnerable population, so it would require an expedited review.
Answer to Question 2
ANS: C
The functions of an IRB involve reviewing research to determine whether the rights and welfare of the subjects were protected, the methods used to secure informed consent were appropriate, and the potential benefits of the study were greater than the risks.
The IRB examines studies for ethical concerns. Their job is to protect human subjects, not critique the research methods. The functions of an IRB involve reviewing research to determine whether the rights and welfare of the subjects were protected, the methods used to secure informed consent were appropriate, and the potential benefits of the study were greater than the risks.
The IRB examines studies for ethical concerns. Their job is to protect human subjects, not design and develop studies. The functions of an IRB involve reviewing research to determine whether the rights and welfare of the subjects were protected, the methods used to secure informed consent were appropriate, and the potential benefits of the study were greater than the risks.
The IRB examines studies for ethical concerns. Their job is to protect human subjects, not reviewing cost associated with research. The functions of an IRB involve reviewing research to determine whether the rights and welfare of the subjects were protected, the methods used to secure informed consent were appropriate, and the potential benefits of the study were greater than the risks.