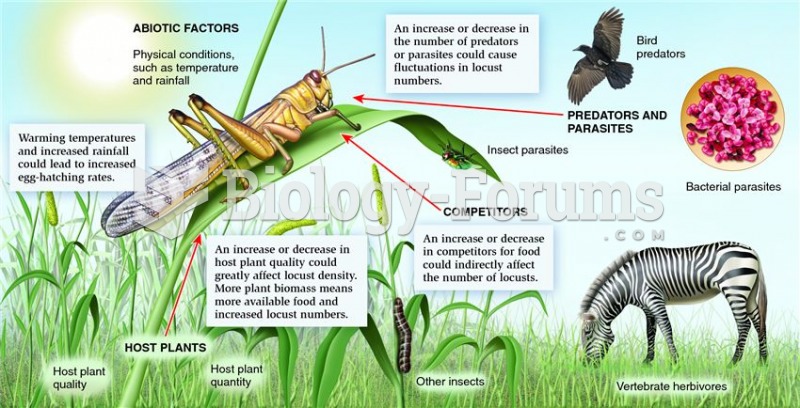
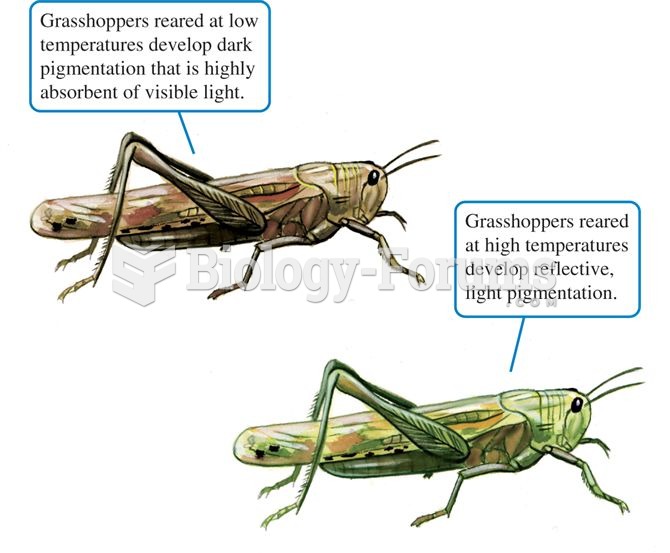
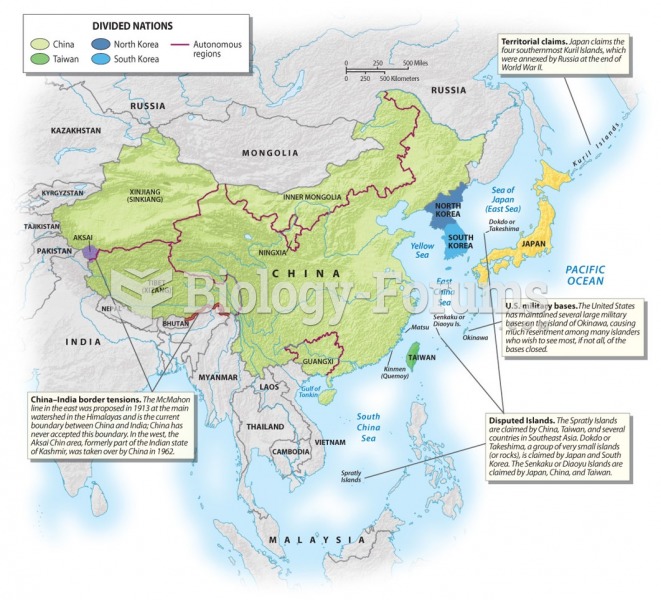
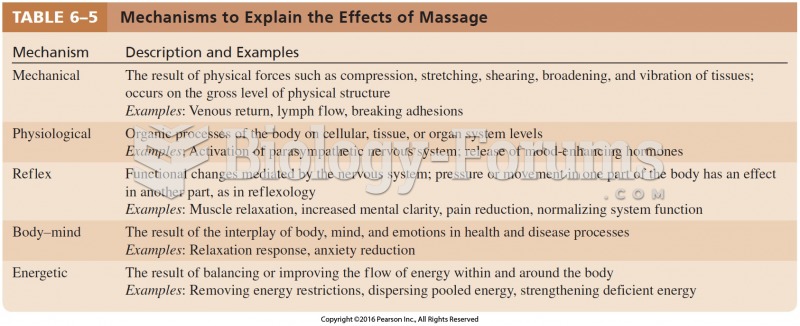
Answer to Question 1
ANSWER: Chlorofluorocarbons (CFCs) posed a serious threat to stratospheric ozone. In the 1970s, chlorofluorocarbons were the most widely used propellants in spray cans, such as deodorants and hairsprays. In the troposphere, these gases are quite safe, being nonflammable, nontoxic, and unable to chemically combine with other substances. Although the use of CFCs has decreased by more than 95 percent, there are still millions of kilograms in the troposphere that will continue to slowly diffuse upward, and thus we will continue to see signs of ozone depletion for years to come.
There are now two major substitutes for CFCs: hydrochlorofluoroca rbons (HCFCs) and hydrofluorocarbons (HFCs). The HCFCs contain fewer chlorine atoms per molecule than CFCs and, therefore, pose much less danger to the ozone layer, whereas HFCs contain no chlorine at all. However, like CFCs, these two groups of substitutes are also greenhouse gases that can enhance global warming. Because of this, the Montreal Protocol now calls for HCFCs to be phased out by 2030, and several nations have proposed phasing out HFCs as well.
Answer to Question 2
ANSWER: In recent decades, much attention has been placed on reducing the depletion of stratospheric ozone because of the many risks such a loss would pose. These include a(n):
1. increase in the number of cases of skin cancer
2. sharp increase in eye cataracts and sunburns
3. suppression of the human immune system
4. adverse impacts on crops and animals due to an increase in ultraviolet radiation
5. reduction in the growth of ocean phytoplankton
6. cooling of the stratosphere that could alter stratospheric wind patterns, possibly affecting the destruction of ozone