|
|
|
The National Institutes of Health have supported research into acupuncture. This has shown that acupuncture significantly reduced pain associated with osteoarthritis of the knee, when used as a complement to conventional therapies.
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
Asthma cases in Americans are about 75% higher today than they were in 1980.
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
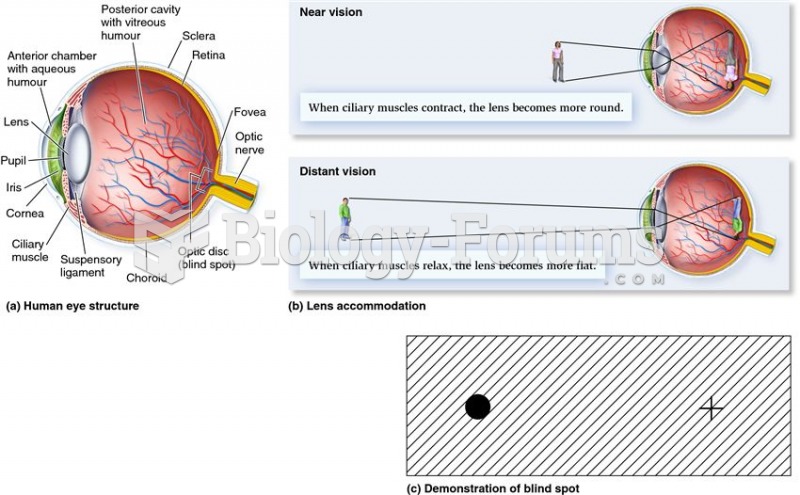
Astigmatism is the most common vision problem. It may accompany nearsightedness or farsightedness. It is usually caused by an irregularly shaped cornea, but sometimes it is the result of an irregularly shaped lens. Either type can be corrected by eyeglasses, contact lenses, or refractive surgery.
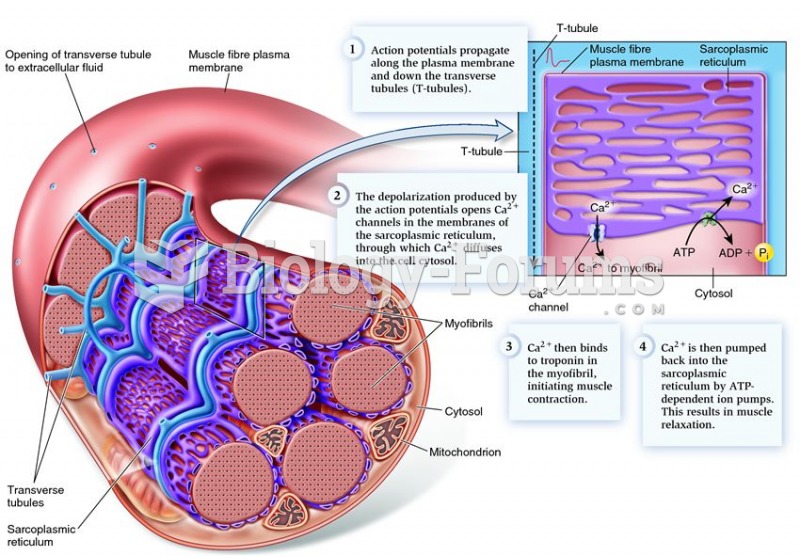 Arrangement of the sarcoplasmic reticulum, transverse tubules, and myofibrils in a single skeletal m
Arrangement of the sarcoplasmic reticulum, transverse tubules, and myofibrils in a single skeletal m
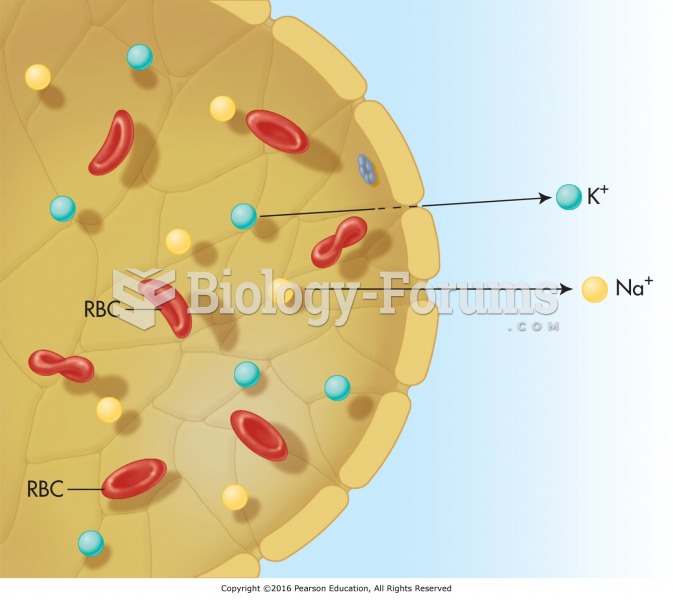 The process of filtration in the kidneys, where smaller solutes, such as the electrolytes sodium and ...
The process of filtration in the kidneys, where smaller solutes, such as the electrolytes sodium and ...