Answer to Question 1
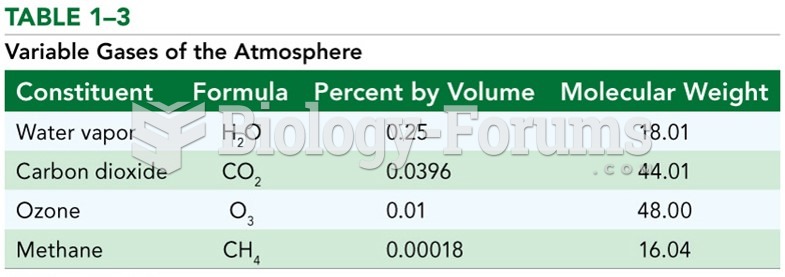
Ozone is a molecule of three oxygen atoms that absorbs ultraviolet energy from incoming solar radiation. In so doing, the ozone molecule is split into individual oxygen atoms. In the process of absorbing UV and splitting apart, the ozone molecule prevents UV from reaching Earth's surface. Once divided, the individual oxygen atoms can re-bond and create a new ozone molecule ready to absorb more incoming UV. Because of its role in shielding the Earth from UV radiation, the ozone layer (found only in the stratosphere) is critical to life on this planet. Unfortunately, scientists identified a hole in the ozone layer several decades ago. Eventually, the scientists Crutzen, Rowland, and Molina were able to identify that this hole was being caused by the presence of CFCs (chlorofluorocarbons) in the atmosphere. CFCs at the time were widely used in a variety of things, including refrigerants, styrofoam, and aerosol cans, because of their excellent thermal properties and long life span. However, the chlorine in CFCs is capable of bonding with ozone in the upper atmosphere and tearing it apart, thus reducing the number of ozone molecules in the ozone layer. The gradual buildup of CFCs in the upper atmosphere caused a hole to form in the ozone. When the world realized that CFCs were the primary cause of the rapidly growing hole in the ozone layer over Antarctica, the international community hastened to enact a treaty which limited and eventually eliminated CFC production and use. This treaty was known as the Montreal Protocol. As CFC production is stopped and existing CFCs work their way out of the system over the next century, it is expected that the ozone layer will recover and the hole will disappear.
Answer to Question 2
FALSE