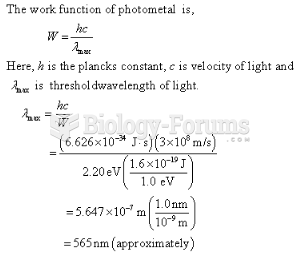
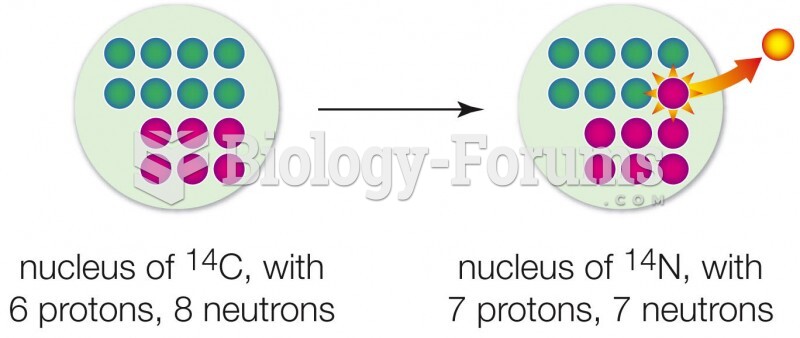
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.
Did you know?
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.
Did you know?
Less than one of every three adults with high LDL cholesterol has the condition under control. Only 48.1% with the condition are being treated for it.
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.