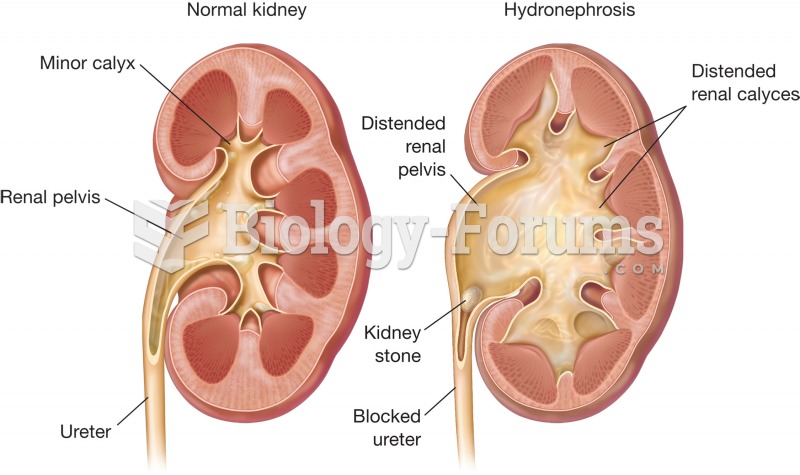
Answer to Question 1
i. Mixing of air masses with different densities.
ii. Uplift due to convection causes heavy precipitation along the polar front zone in the mid-latitudes and in the vicinity of the Inter-Tropical Convergence Zone (ITCZ). Convection occurs not only in the tropics, but wherever there is intense surface heating. Therefore, although convection does not always produce rain, it is the dominant rainfall-producing process over warm land masses in summer.
iii. An air mass moving across a mountain range can cause uplift from the increasing elevation of the land surface.
Answer to Question 2
Once the rate of condensation equals the evaporation rate, the gas is at equilibrium. At this point, the vapor pressure of water is referred to as the saturation vapor pressure. In this scenario, the saturation vapor pressure depends only on the rate at which molecules are transferred from liquid to gas and back again.
a. Draw a graph that plots saturation vapor pressure as a function of vapor pressure and temperature.
b. Explain why the information shown in the plot is useful for understanding the relationships between atmospheric circulation and precipitation.
The figure above is a graph of saturation vapor pressure versus temperature for water. In general, we can think of clouds as forming when the air is at the saturation vapor pressure for water. Further evaporation adds water vapor molecules to the air, where they condense to form water droplets in clouds. When these droplets become large enough to overcome the upward motion of the air, they fall as precipitation. Assume that an air mass is at the temperature and vapor pressure indicated by point P in this figure. Point P is not on the curve; the air is not at the saturation vapor pressure for that temperature, hence clouds would not form and precipitation could not occur. We can bring that air mass to the saturation pointthat is, to equilibrium at a point on the temperature versus saturation vapor pressure curvein two ways. First, we could add more water vapor through increased evaporation from the surface and thereby increase the saturation vapor pressure. That action would move the air mass from point P up along the vertical dashed line toward the curve. Second, we could reduce the air temperature, which would move the air mass from point P to the left along the horizontal dashed line toward the curve. So this curve shows that the vapor pressure can be brought to the saturation point either by increasing the vapor pressure or by cooling the air.