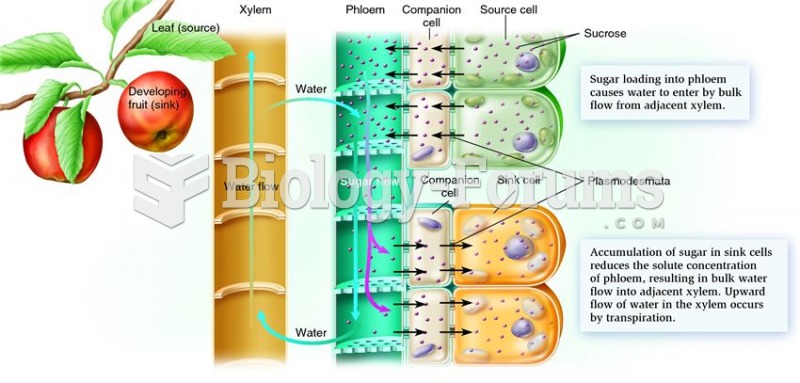
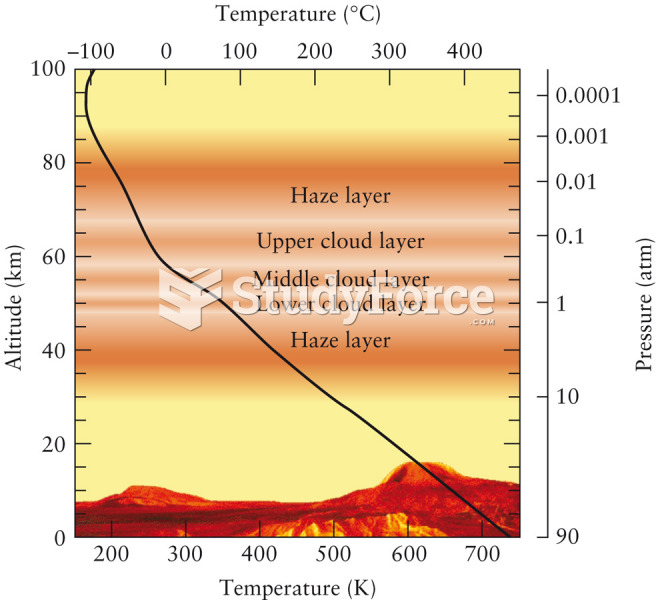
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
Did you know?
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Did you know?
Vaccines cause herd immunity. If the majority of people in a community have been vaccinated against a disease, an unvaccinated person is less likely to get the disease since others are less likely to become sick from it and spread the disease.
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.
Did you know?
According to animal studies, the typical American diet is damaging to the liver and may result in allergies, low energy, digestive problems, and a lack of ability to detoxify harmful substances.