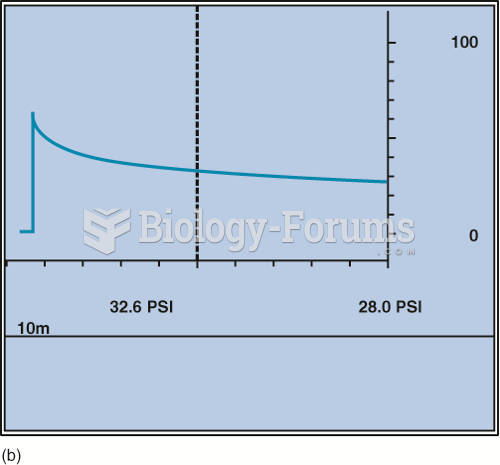
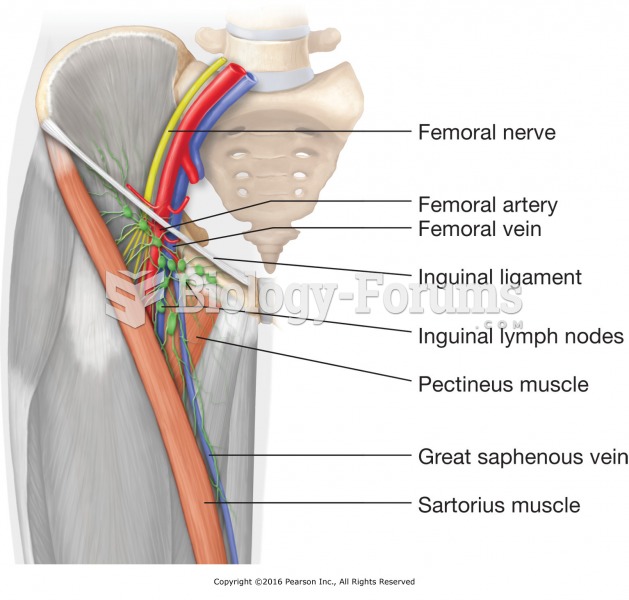
|
|
|
Blood is approximately twice as thick as water because of the cells and other components found in it.
Looking at the sun may not only cause headache and distort your vision temporarily, but it can also cause permanent eye damage. Any exposure to sunlight adds to the cumulative effects of ultraviolet (UV) radiation on your eyes. UV exposure has been linked to eye disorders such as macular degeneration, solar retinitis, and corneal dystrophies.
Ether was used widely for surgeries but became less popular because of its flammability and its tendency to cause vomiting. In England, it was quickly replaced by chloroform, but this agent caused many deaths and lost popularity.
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
The human body produces and destroys 15 million blood cells every second.