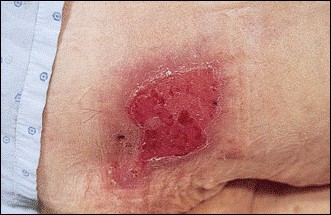
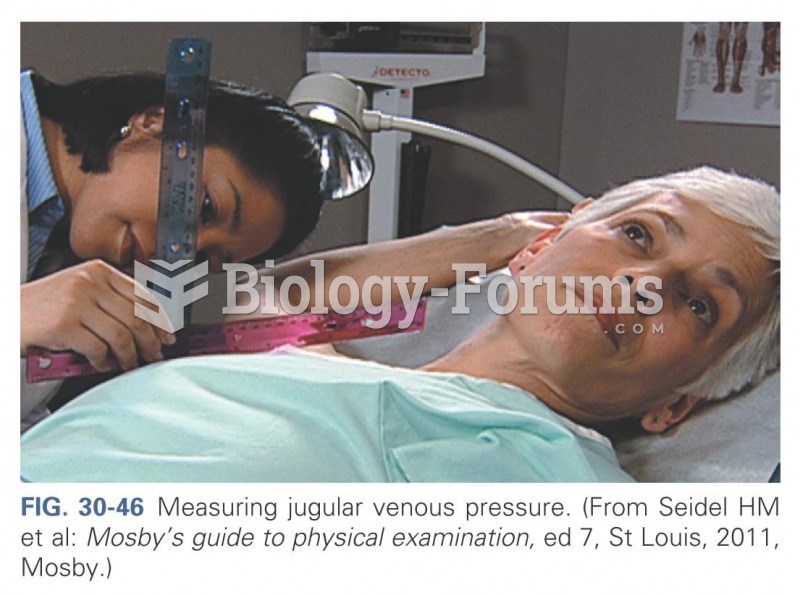
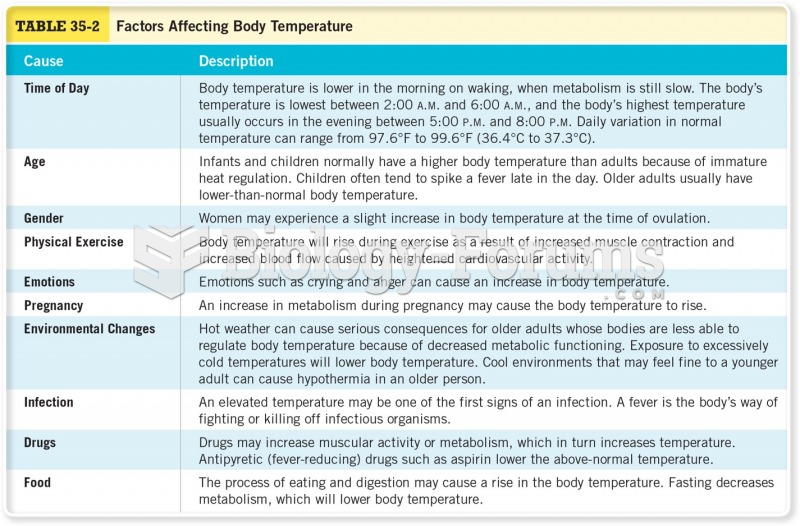
|
|
|
Vaccines prevent between 2.5 and 4 million deaths every year.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
Bisphosphonates were first developed in the nineteenth century. They were first investigated for use in disorders of bone metabolism in the 1960s. They are now used clinically for the treatment of osteoporosis, Paget's disease, bone metastasis, multiple myeloma, and other conditions that feature bone fragility.
The effects of organophosphate poisoning are referred to by using the abbreviations “SLUD” or “SLUDGE,” It stands for: salivation, lacrimation, urination, defecation, GI upset, and emesis.