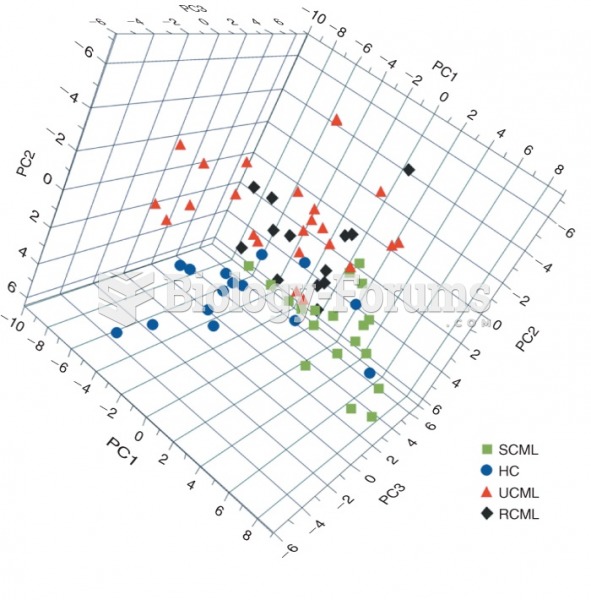
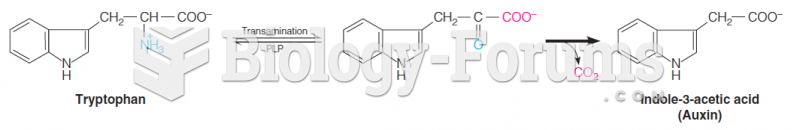
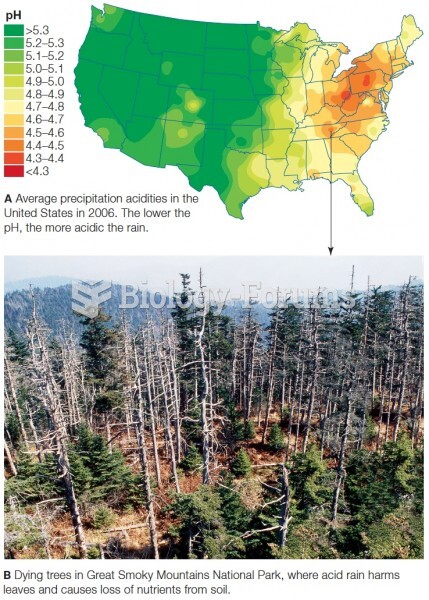
|
|
|
Did you know?
Illness; diuretics; laxative abuse; hot weather; exercise; sweating; caffeine; alcoholic beverages; starvation diets; inadequate carbohydrate consumption; and diets high in protein, salt, or fiber can cause people to become dehydrated.
Did you know?
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.