|
|
|
Of the estimated 2 million heroin users in the United States, 600,000–800,000 are considered hardcore addicts. Heroin addiction is considered to be one of the hardest addictions to recover from.
A recent study has found that following a diet rich in berries may slow down the aging process of the brain. This diet apparently helps to keep dopamine levels much higher than are seen in normal individuals who do not eat berries as a regular part of their diet as they enter their later years.
There are 20 feet of blood vessels in each square inch of human skin.
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
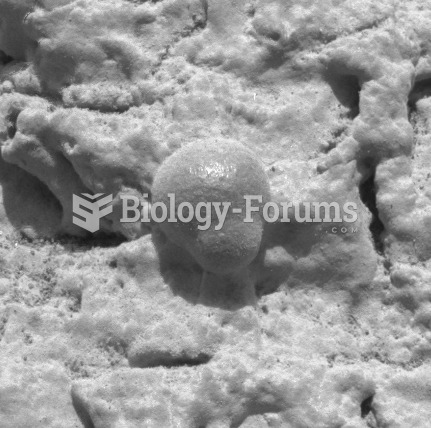 Microscopic photo taken by Opportunity showing a gray hematite concretion, indicative of the past pr
Microscopic photo taken by Opportunity showing a gray hematite concretion, indicative of the past pr
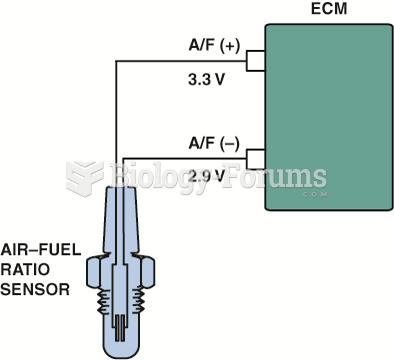
 Checking the 5 volt reference from the computer being applied to the TP sensor with the ignition ...
Checking the 5 volt reference from the computer being applied to the TP sensor with the ignition ...