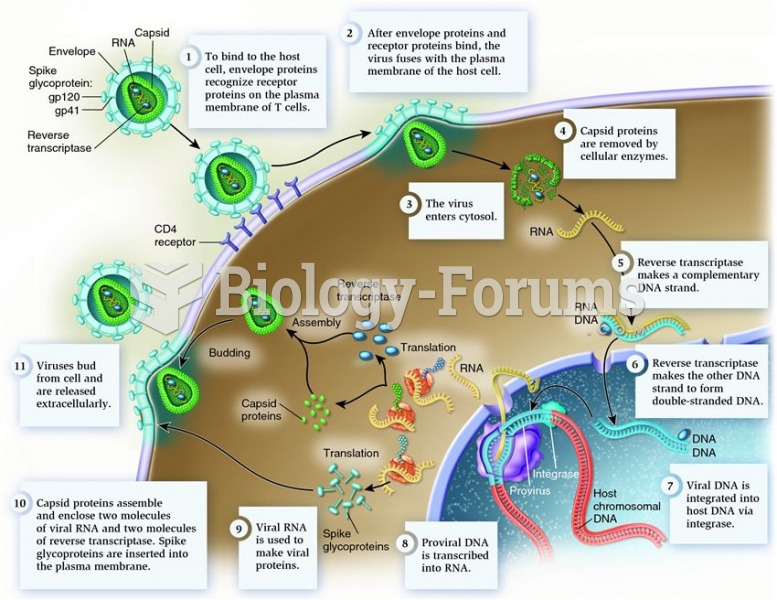
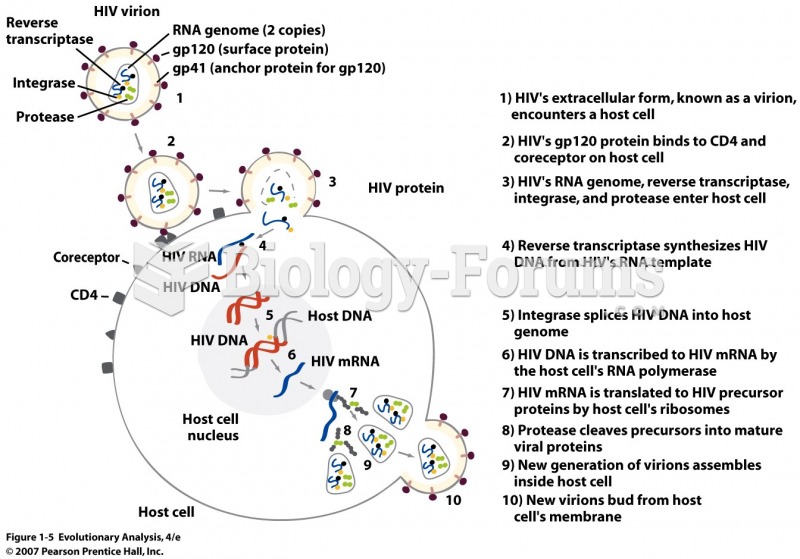
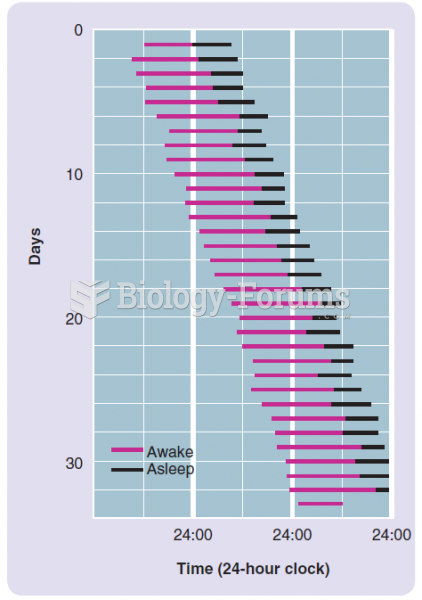
Answer to Question 1ANS:Of
the essential elements, nitrogen undergoes the most movement and change. The series of gains,
losses, and changes is termed the nitrogen cycle. The central portion of the nitrogen cycle operates
through the action of soil microorganisms. To review briefly, nitrogen comes from nitrogen gas in the
atmosphere. Symbiotic or nonsymbiotic bacteria use that nitrogen to form protein for their own bodies
or supply it to host plants. When these bacteria or host plants die, other microbes mineralize the
protein into ammonium ions. This particular case of mineralization is termed ammonification.
Ammonium nitrogen can be taken up by plants, but most is converted by bacteria to nitrite ions and
then to nitrate ions. Nitrates are taken up by plants or microbes or return to the atmosphere as nitrogen
gas through the process of denitrification.
The complete nitrogen cycle includes some nonbiological processes as well. Two other forms of
fixation add usable nitrogen to the soil. First, lightning during storms provides energy to combine
gaseous nitrogen and oxygen to form nitrogen dioxide. The gas dissolves in water vapor to produce
nitric acid. Second, large amounts of nitrogen are fixed from the air in fertilizer factories and applied
to soil by growers.
Two nonbiological losses of nitrogen from soil may be important. The nitrate ion is negatively charged
and not adsorbed by soil colloids. Nor is it held in soil by other means, so nitrates easily leach from the
soil. Although ammonia does not leach readily, it can be lost by a process called ammonia
volatilization. The smell of an open bottle of household ammonia (ammonia gas dissolved in water) is
a result of this reaction. Substantial losses of ammonium nitrogen can occur when ammonium
fertilizers or manure are applied to soil, especially if it is alkaline or recently limed.
In native habitats, including virgin forests or prairies, gains and losses in the cycle balance over time.
Growing crops (or even turf lawns) changes the balance greatly in ways that increase nitrogen losses:Nitrogen
is removed by harvest, or removal of grass clippings from a lawn
Nitrogen is often applied heavily in excess of plant needs; this excess will not be
taken up by plants and is free to move
Cropped soil is more likely to erode, so nitrogen and other nutrients are carried off in
running water.
Irrigation increases percolation of water through the soil profile, increasing nitrate
losses by leaching. At the same time, wetter soil may increase denitrification losses.
Liming may increase the loss of ammonia by volatilization.
To compensate for increased nitrogen losses and to meet the needs of modern high productivity,
growers supply more nitrogen by manuring, growing legumes, or by fertilization. The trend away from
a mixed farming operation toward one that specializes in either cash crops or raising animals improves
economic efficiency, but exacts a penalty in view of the nitrogen cycle. For the cash crop grower, more
money must be spent on fertilizers. For the animal raiser, manure becomes a waste disposal problem.
Answer to Question 2ANS: Molybdenum