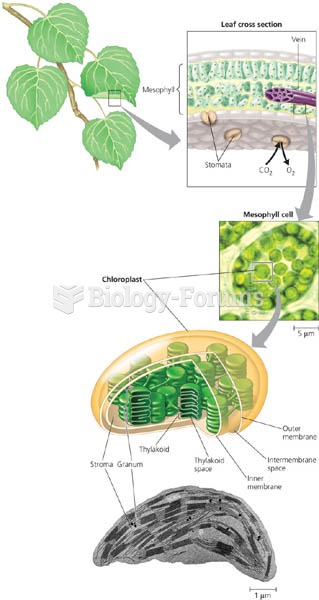
Answer to Question 1T
Answer to Question 2Saline soils have high levels of soluble salts except sodium. Soil salinity is measured by
passing an electrical current through a solution extracted from a soil sample. The greater the salt
content, the greater the electrical current. The value is called electrical conductivity (EC). A saline soil
has electrical conductivity of four or more millimhos per centimeter. However, even lower salinity
levels can injure sensitive plants. Most salts are chlorides or sulfates. Less than half of the cations are
sodium, and little sodium is adsorbed on soil colloids. Soils can be classified for use based on salinity.
Sodic soils are lower in the kinds of salts found in saline soils, but high in sodium. The
exchangeable sodium percentage is 15 or more, and pH is in the range 8.5 to 10.0. Sodium is often
measured by the sodium adsorption ratio (SAR). The SAR compares concentration of sodium ions
with the concentration of calcium and magnesium ions. Using this measurement, a sodic soil has an
SAR greater than or equal to 13. Sodic soil has a number of effects on plant growth:Sodium
reacts with water to form lye. The resulting high pH limits growth of most plants.
Sodium can destroy soil structure. Sodium ions saturate cation exchange sites and colloids
separate and disperse soil aggregates. These tiny soil particles lodge in the soil pores,
sealing the soil surface and creating wet slick spots. Tilth suffers and crusts hard enough
to stop seed germination may form. Sodic soils may also show a poorly drained columnar
subsoil structure.
Plants may take up enough sodium to injure plant tissues.
Saline-sodic soils contain high levels of both soluble salts and sodium. The physical structure
of these soils is normal. However, after periods of heavy rain or irrigation with low-salt water, soluble
calcium and magnesium may leach out of the soil, leaving behind sodium salts. Soil may then become
sodic, with poor physical structure and drainage.