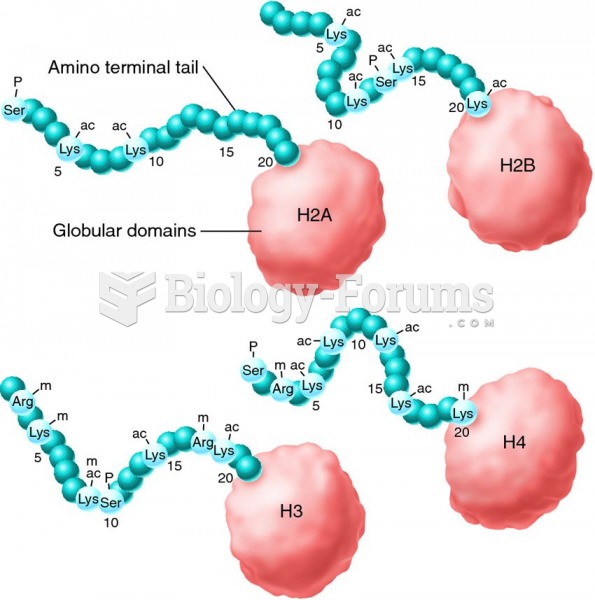
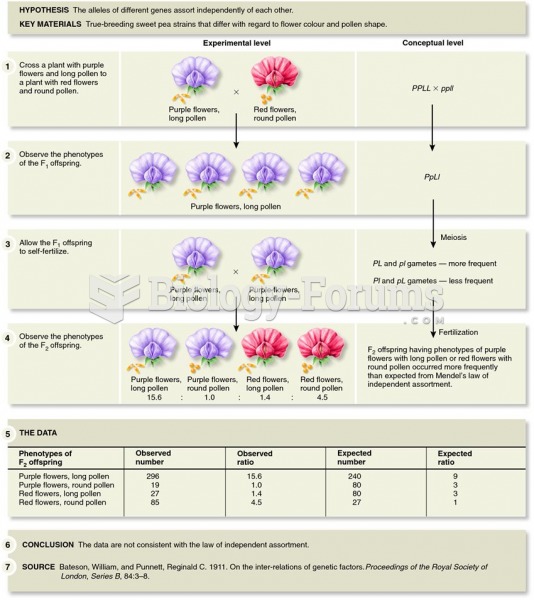
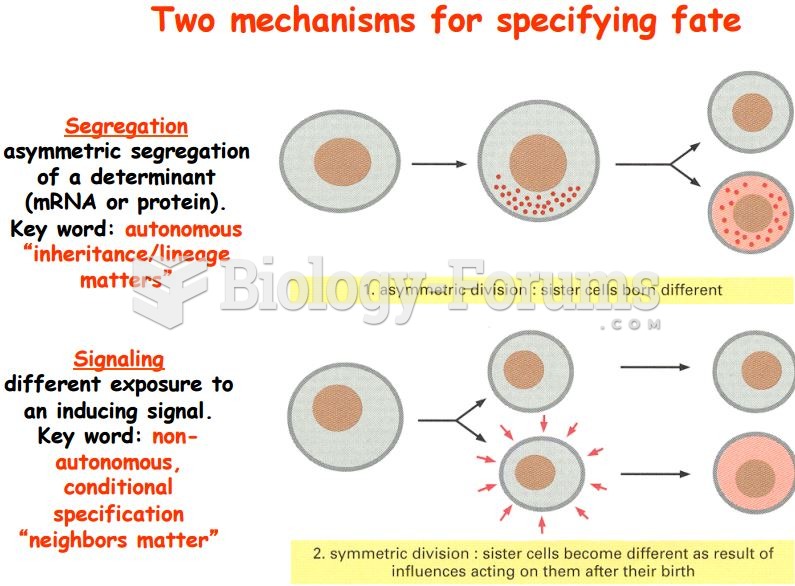
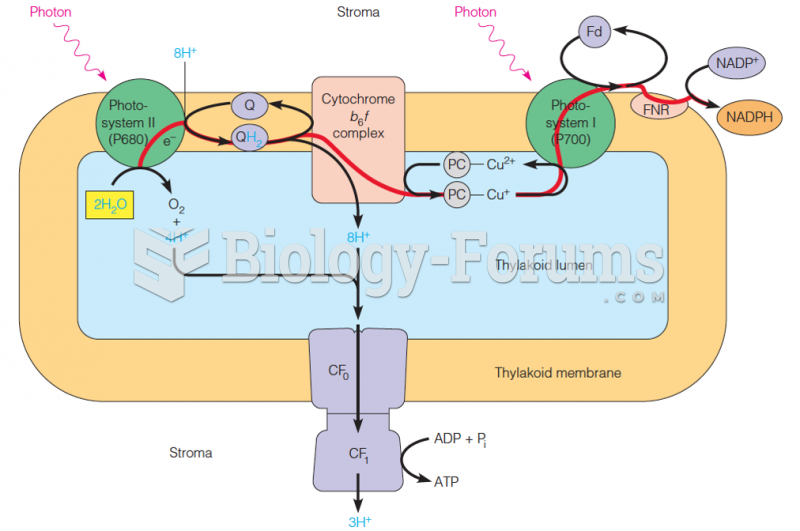
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Eating food that has been cooked with poppy seeds may cause you to fail a drug screening test, because the seeds contain enough opiate alkaloids to register as a positive.
Did you know?
Adult head lice are gray, about ? inch long, and often have a tiny dot on their backs. A female can lay between 50 and 150 eggs within the several weeks that she is alive. They feed on human blood.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
There are more bacteria in your mouth than there are people in the world.
Did you know?
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.