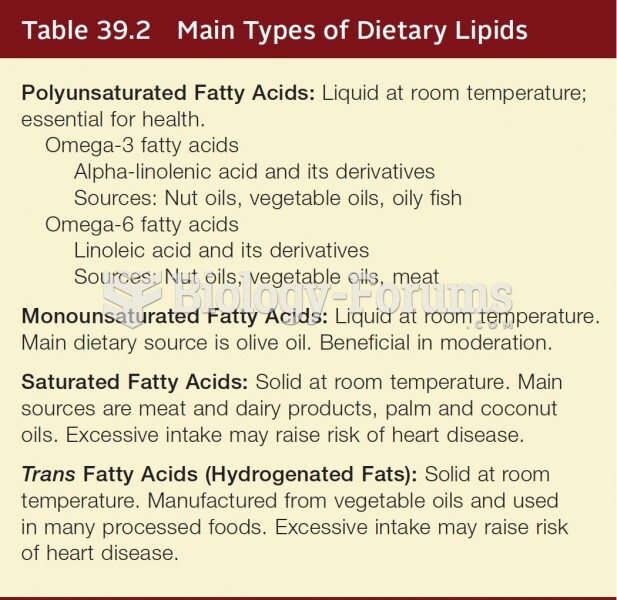
Answer to Question 1
Correct Answer: 1
Rationale 1: Exclusivity allows a pharmaceutical company a period of time to recoup the costs of research and development of a drug.
Rationale 2: The period of exclusivity is not granted so that consumers will become familiar with a trade name.
Rationale 3: Adverse effects are discovered during the clinical drug trials, not during the period of exclusivity.
Rationale 4: Competition between pharmaceutical companies actually results in consumer savings.
Global Rationale: Exclusivity allows a pharmaceutical company a period of time to recoup the costs of research and development of a drug. Exclusivity is not granted so that consumers will become familiar with a trade name. Adverse effects are discovered during the clinical drug trials, not during the period of exclusivity. Competition between pharmaceutical companies actually results in consumer savings.
Answer to Question 2
Correct Answer: 4
Rationale 1: While the cost of the trade version is usually greater than that of the generic version of the same drug, cost does not affect bioavailability.
Rationale 2: The time of onset of action is not always an issue in using the generic over the trade version.
Rationale 3: The difference in inert ingredients is not always an issue in substitution of a generic over the trade version.
Rationale 4: The nurse should not substitute a generic drug for a trade version if the drug is a critical care drug or has a narrow safety margin.
Global Rationale: The nurse should not substitute a generic drug for a trade version if the drug is a critical care drug or has a narrow safety margin. While the cost of the trade version is usually greater than that of the generic version of the same drug, cost does not affect bioavailability. The time of onset of action is not always an issue in using the generic over the trade version. The difference in inert ingredients is not always an issue in substitution of a generic over the trade version.