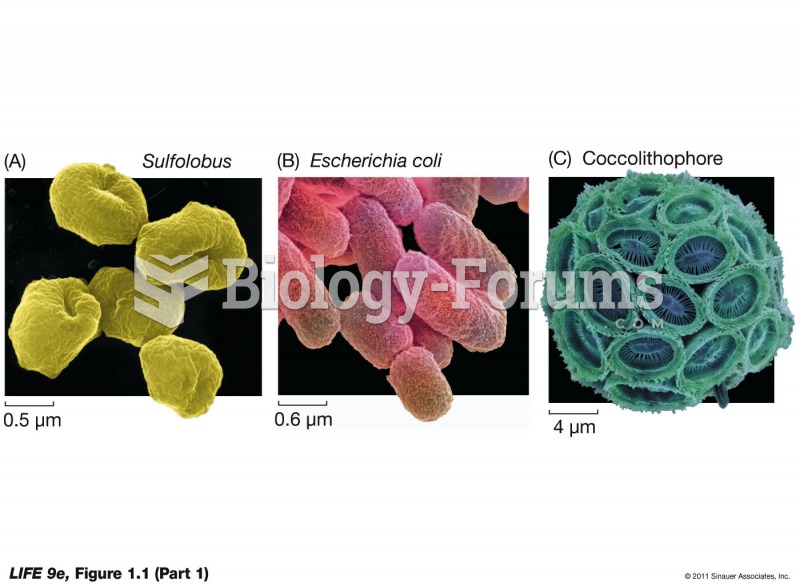
Answer to Question 1
No, it is hypothesized that Earth's early atmosphere contained methane, hydrogen, ammonia and some water vapor. Oxygen came later, and in fact, there is oxygen in today's atmosphere because of life. Stromatolites and other photosynthetic organisms would have begun adding oxygen, a product of photosynthesis, to Earth's early atmosphere. Oxygen tends to disappear from the atmosphere almost as soon as it is released because it readily combines with iron in the soil and ocean water. Geological evidence indicates that Earth's surface iron became saturated with oxygen about 2 to 2.5 billion years ago, after which the proportion of oxygen in the atmosphere began steadily increasing. Oxygen metabolism produces much more energy per mass of food than other reactions, and biologists speculate that this greater efficiency allowed for the development of multicelled organisms at about that same time. Also, an oxygen abundance of only 0.1 percent would have created an ozone screen, protecting organisms from the Sun's ultraviolet radiation and later allowing life to colonize the land.
Answer to Question 2
An important experiment performed by Stanley Miller and Harold Urey in 1952 sought to re-create the presumed conditions in which life on Earth began. The Miller-Urey experiment consisted of a sterile, sealed glass container holding water, hydrogen, ammonia, and methane thought to resemble the young Earth's atmosphere. An electric arc inside the apparatus made sparks to simulate the effects of lightning. Miller and Urey let the experiment run for a week and then analyzed the material inside. They found that the interaction between the electric arc and the simulated atmosphere had produced many organic molecules from the raw material of the experiment, including such important building blocks of life as amino acids. When the experiment was run again using different energy sources such as hot silica to represent molten lava spilling into the ocean, similar molecules were produced. Even a source of ultraviolet radiation representing the amount of UV in sunlight was sufficient to produce complex organic molecules.