This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Did you know?
Adults are resistant to the bacterium that causes Botulism. These bacteria thrive in honey – therefore, honey should never be given to infants since their immune systems are not yet resistant.
Did you know?
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
Did you know?
Your heart beats over 36 million times a year.
Did you know?
More than one-third of adult Americans are obese. Diseases that kill the largest number of people annually, such as heart disease, cancer, diabetes, stroke, and hypertension, can be attributed to diet.
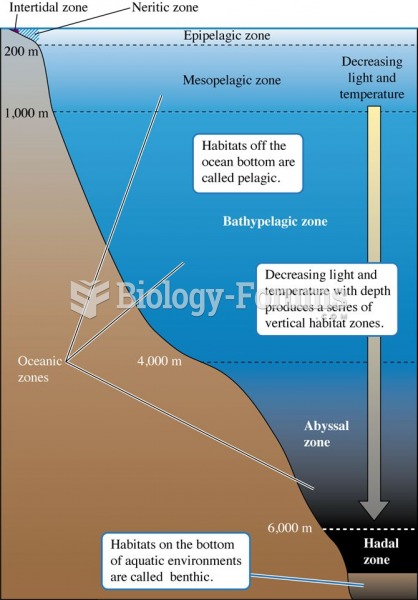 Vertical structuring of the oceans is associated with substantial variation in light and temperature
Vertical structuring of the oceans is associated with substantial variation in light and temperature
 Blue light is scattered more than other wavelengths by the gases in the atmosphere, giving the Earth
Blue light is scattered more than other wavelengths by the gases in the atmosphere, giving the Earth
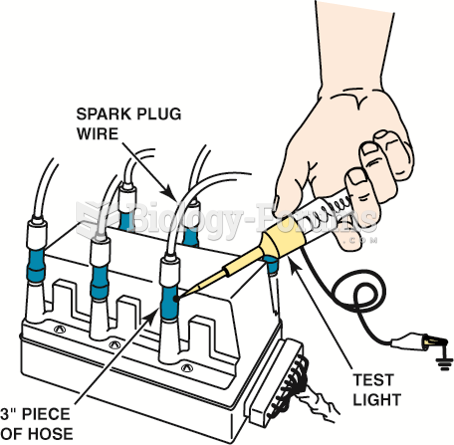 Using a vacuum hose and a test light to ground one cylinder at a time on a distributorless ignition ...
Using a vacuum hose and a test light to ground one cylinder at a time on a distributorless ignition ...