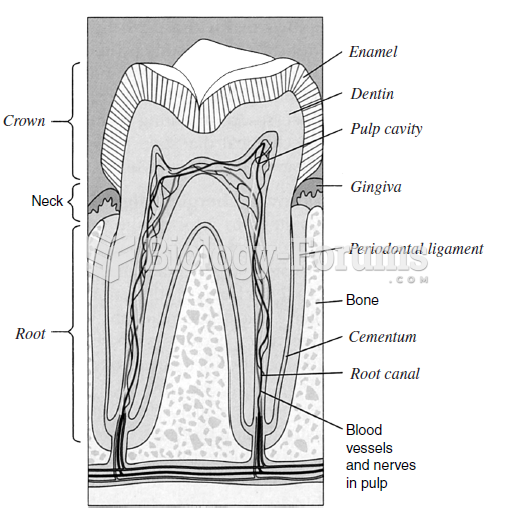
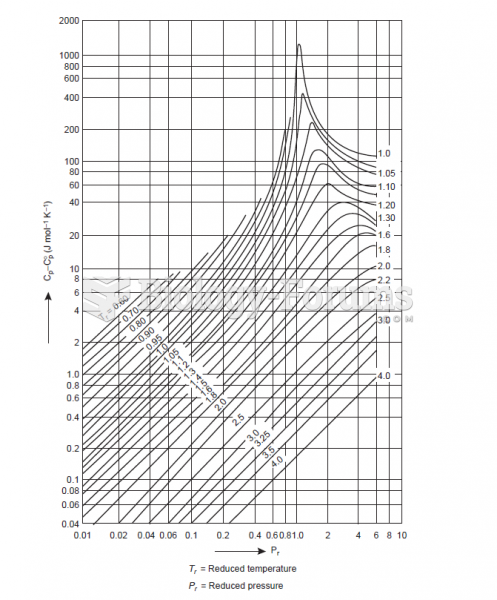
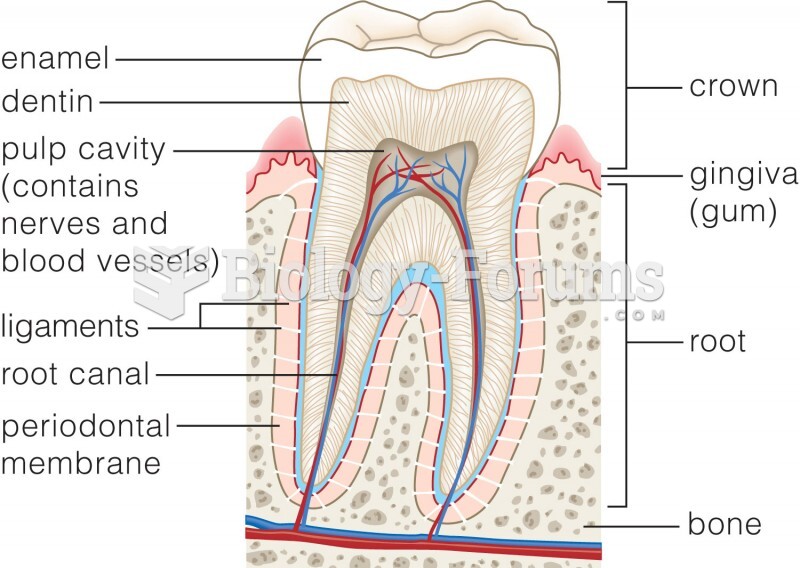
|
|
|
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
Bisphosphonates were first developed in the nineteenth century. They were first investigated for use in disorders of bone metabolism in the 1960s. They are now used clinically for the treatment of osteoporosis, Paget's disease, bone metastasis, multiple myeloma, and other conditions that feature bone fragility.
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.
There are major differences in the metabolism of morphine and the illegal drug heroin. Morphine mostly produces its CNS effects through m-receptors, and at k- and d-receptors. Heroin has a slight affinity for opiate receptors. Most of its actions are due to metabolism to active metabolites (6-acetylmorphine, morphine, and morphine-6-glucuronide).
Drugs are in development that may cure asthma and hay fever once and for all. They target leukotrienes, which are known to cause tightening of the air passages in the lungs and increase mucus productions in nasal passages.