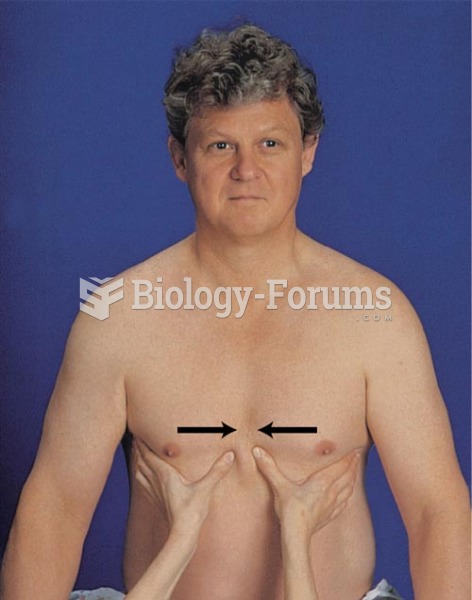
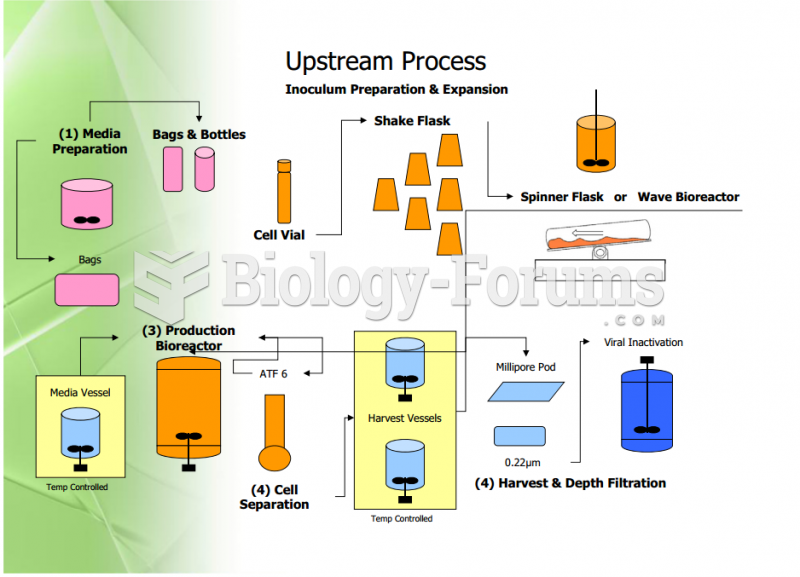
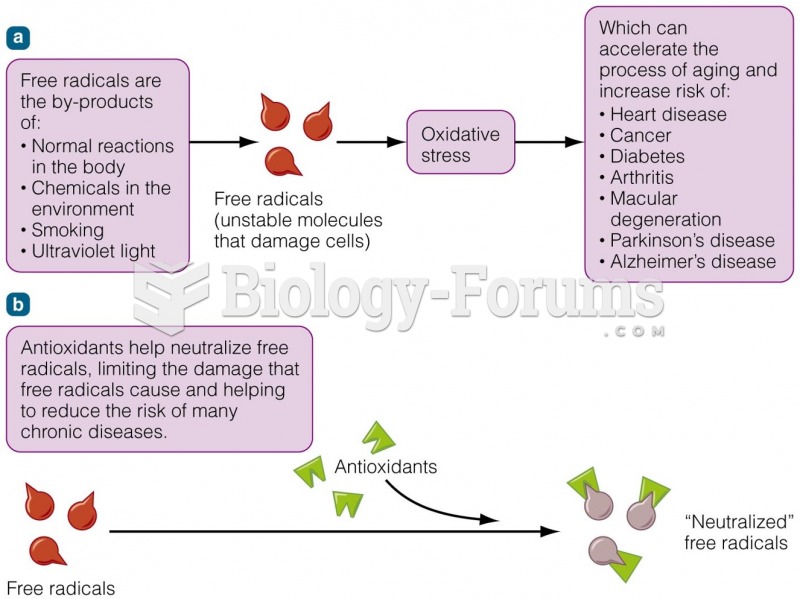
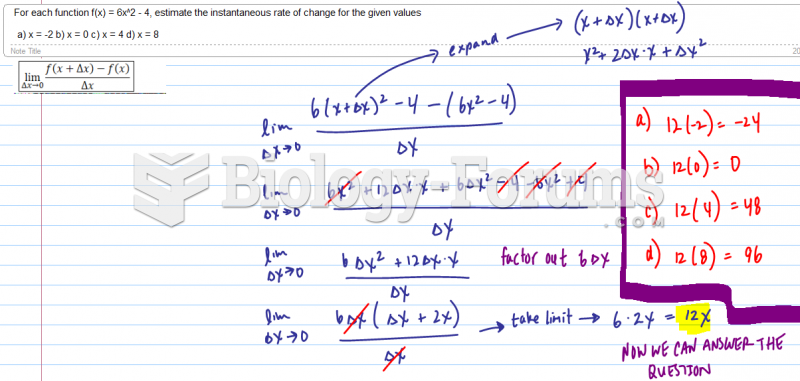
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In 1835 it was discovered that a disease of silkworms known as muscardine could be transferred from one silkworm to another, and was caused by a fungus.
Did you know?
The human body produces and destroys 15 million blood cells every second.
Did you know?
Patients who cannot swallow may receive nutrition via a parenteral route—usually, a catheter is inserted through the chest into a large vein going into the heart.
Did you know?
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
Did you know?
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.