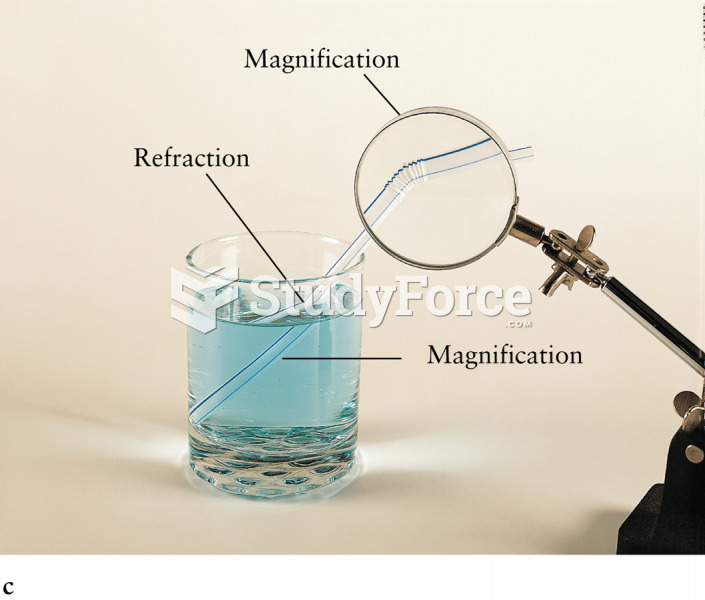
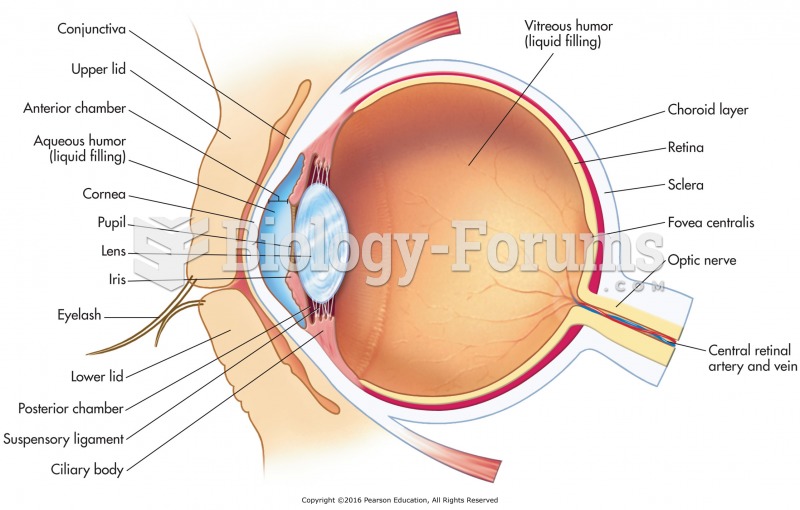
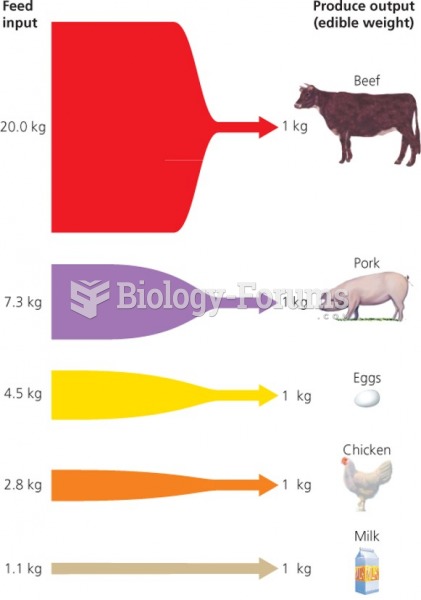
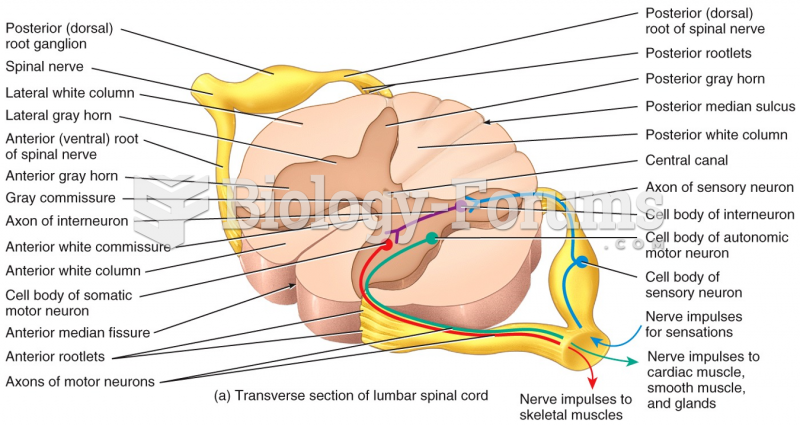
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Though the United States has largely rejected the metric system, it is used for currency, as in 100 pennies = 1 dollar. Previously, the British currency system was used, with measurements such as 12 pence to the shilling, and 20 shillings to the pound.
Did you know?
Calcitonin is a naturally occurring hormone. In women who are at least 5 years beyond menopause, it slows bone loss and increases spinal bone density.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
Asthma occurs in one in 11 children and in one in 12 adults. African Americans and Latinos have a higher risk for developing asthma than other groups.
Did you know?
Cyanide works by making the human body unable to use oxygen.