|
|
|
Excessive alcohol use costs the country approximately $235 billion every year.
Adolescents often feel clumsy during puberty because during this time of development, their hands and feet grow faster than their arms and legs do. The body is therefore out of proportion. One out of five adolescents actually experiences growing pains during this period.
Stroke kills people from all ethnic backgrounds, but the people at highest risk for fatal strokes are: black men, black women, Asian men, white men, and white women.
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
IgA antibodies protect body surfaces exposed to outside foreign substances. IgG antibodies are found in all body fluids. IgM antibodies are the first type of antibody made in response to an infection. IgE antibody levels are often high in people with allergies. IgD antibodies are found in tissues lining the abdomen and chest.
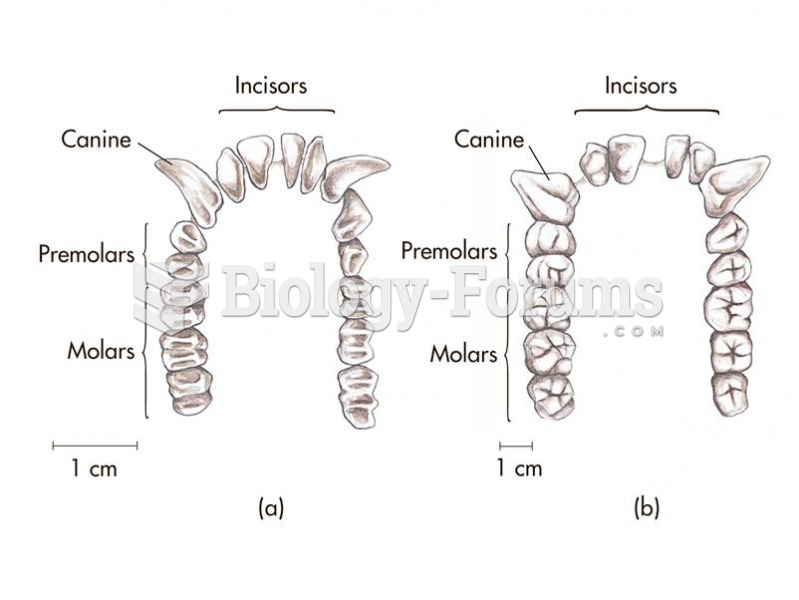 The primate dental formula illustrated for (a) the lower dentition of an Old World monkey and (b) th
The primate dental formula illustrated for (a) the lower dentition of an Old World monkey and (b) th
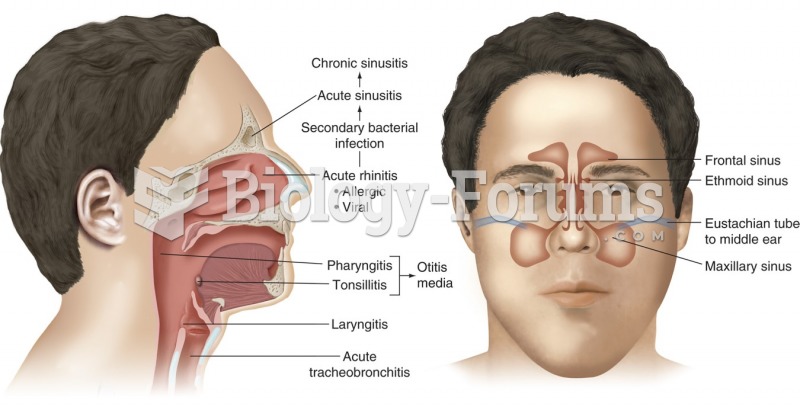 Paranasal sinuses are part of the upper respiratory system. From here, infections may spread via the ...
Paranasal sinuses are part of the upper respiratory system. From here, infections may spread via the ...